ThyroKare Tablets
This treatment applies to the following species: Company: Neogen
Company: Neogen
(levothyroxine sodium tablets), USP
For oral use in dogs only
Before using this drug, read package insert for full prescribing information.
ThyroKare Tablets Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
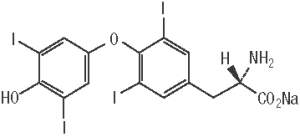
ThyroKare (levothyroxine sodium tablets), USP contains synthetic L-3,3’,5,5’-tetraiodothyronine sodium salt [levothyroxine (T4) sodium]. Synthetic levothyroxine sodium is identical to the hormone produced in the thyroid gland of a dog. Levothyroxine sodium has an empirical formula of C15H10I4NNaO4 • H2O, molecular weight of 798.85 g/mol (anhydrous) and structural formula as shown:
INDICATION: For replacement therapy for diminished thyroid function in dogs.
Dosage and Administration
The initial dose is 0.1 mg/10 lb (0.01 mg/lb, 0.022 mg/kg) body weight twice daily. To minimize day-to-day variations in serum total thyroxine (tT4) concentrations (see CLINICAL PHARMACOLOGY), owners should consistently administer ThyroKare either with or without food. To maintain serum levothyroxine sodium concentrations over time, therapeutic monitoring should be conducted every 4 weeks until an adequate maintenance dose is established and then as needed for continued maintenance.When switching from another levothyroxine sodium formulation to ThyroKare, monitor serum tT4 concentrations and clinical response due to potential differences in recommended starting doses and potential differences in bioavailability.
Contraindications
Do not use in dogs with thyrotoxicosis, acute myocardial infarction, or uncorrected adrenal insufficiency.
Warnings
User Safety Warnings: Not for human use. Keep out of reach of children. In the event of accidental ingestion, seek medical advice immediately and show the product label to the physician. Wash hands after handling.
Animal Safety Warnings: Keep ThyroKare in secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
Precautions
Use with caution in dogs with clinically significant cardiovascular disease, diabetes mellitus, or other conditions for which an increased metabolic rate might prove hazardous. Dogs with underlying cardiovascular disease that are diagnosed with hypothyroidism should be closely monitored during the dose establishment phase. Adjustment of cardiovascular medications or levothyroxine sodium dosage may be needed.1-4 The safety of ThyroKare has not been evaluated in breeding, pregnant, or lactating dogs.Adverse Reactions
In a 6-month US US field study, the 120 dogs enrolled in the study receiving a minimum of one dose of ThyroKare were evaluated for safety. The percentage of dogs experiencing adverse reactions is presented in Table 1.Table 1. Percentage of dogs experiencing adverse reactions
|
Adverse Reaction |
Percent |
|
Polydipsia |
30.8 |
|
Polyuria |
20.0 |
|
Tachypnea |
16.7 |
|
Lethargy |
11.7 |
|
Anorexia |
10.0 |
|
Emesis |
10.0 |
|
Muscle tremor/shaking |
10.0 |
|
Hyperactivity |
8.3 |
|
Anxiety |
5.8 |
|
Desquamation/scaling/seborrhea |
5.8 |
|
Diarrhea |
5.0 |
|
Polyphagia/increased appetite |
5.0 |
|
Alopecia and increased shedding |
4.2 |
|
Otitis externa and otorrhea |
4.2 |
|
Increased serum alanine aminotransferase (ALT) |
4.2 |
|
Increased serum alkaline phosphatase (ALP) |
3.3 |
|
Pruritus, including pinnal |
3.3 |
|
Tachycardia |
3.3 |
|
Aggression |
1.7 |
|
Dermatitis and eczema |
1.7 |
|
Lymphopenia |
1.7 |
|
Temporal muscle atrophy |
1.7 |
|
Weight loss |
1.7 |
|
Adipsia |
0.8 |
|
Hypersalivation |
0.8 |
|
Hypersensitivity reaction |
0.8 |
Clinical pathology findings were consistent with stimulation of hematopoiesis as a result of replacement therapy with ThyroKare. However, hematocrit and red blood cell counts exceeded the upper limit of the reference range in 6 dogs at the end of the study; 3 of these dogs also had elevated reticulocyte counts. Nine (9) dogs had transient elevations in neutrophil counts exceeding the reference range at Day 28 that resolved by Day 56. Liver enzyme elevations associated with ThyroKare returned to the reference range by Day 168 in 2 of 4 dogs with increased ALP and 4 of 5 dogs with increased ALT, respectively.
One dog was withdrawn from the study at the owner’s request because of an elevated tT4 concentration and abnormal behavior. A second dog was removed from the study by request of the investigator due to anemia.
A dog with preexisting hypoalbuminemia exhibited declining serum albumin and total protein concentrations concurrently with prolonged elevated serum tT4 concentrations. Although reducing the ThyroKare dose resulted in serum tT4 levels in the therapeutic range, the dog experienced a serious adverse event that included marked weight loss, hypoalbuminemia, hypoproteinemia, elevated ALP, and hypoglycemia. The dog received supportive veterinary care and completed the study while remaining on the same ThyroKare dose. Serum albumin returned to near baseline and total protein normalized by the end of the study.
Twenty-two (22) individual case reports describing 42 adverse reactions related to the clinical use of ThyroKare in dogs were reported voluntarily to Neogen Corporation (as of 2020). The following adverse events were reported: panting, anxiety, elevated or low serum tT4 concentrations, vomiting, diarrhea, lethargy, unspecified skin issues, folliculitis, hyperpigmentation, hair loss, hiding, polyuria, polydipsia, tachycardia, masseter and temporal muscle atrophy, reduced appetite, polyphagia, and seizure.
CONTACT INFORMATION: For a copy of the Safety Data Sheet (SDS) or to report suspected adverse drug events, contact Neogen Corporation at 800.525.2022. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1.888.FDA.VETS or www.fda.gov/reportanimalae.
Clinical Pharmacology
Synthetic levothyroxine (T4) is chemically identical to the naturally-occurring thyroxine hormone. Levothyroxine sodium acts, as does endogenous thyroxine, to stimulate metabolism, growth, development, and differentiation of tissues. Levothyroxine sodium is absorbed rapidly from the gastrointestinal tract after oral administration. When ThyroKare (levothyroxine sodium tablets), USP was administered as a single oral dose of 0.1 mg/10 lb (0.022 mg/kg) to 7 thyroidectomized, fasted Beagles, the absolute bioavailability of levothyroxine sodium was low (19%). After 7 days of twice daily dosing, peak serum tT4 concentrations were reached within 1.3 to 4 hours. The mean (± standard deviation) terminal phase half-life was 9.6 (±3.4) hours. Administration of levothyroxine sodium with food reduces oral bioavailability.5 Levothyroxine sodium is excreted in the feces. Absorption and metabolism of levothyroxine can vary greatly between individual dogs, so therapeutic monitoring of serum tT4 levels is recommended (see DOSAGE AND ADMINISTRATION).Effectiveness
In a US field study, 120 dogs were administered an initial dose of 0.1 mg/10 lb (0.01 mg/lb, 0.022 mg/kg) body weight, given twice daily. The dose could be increased or decreased (without a change in frequency) after 4 and 8 weeks and at unscheduled visits, based on clinical findings and serum thyroid (tT4) hormone concentrations.Treatment success was determined at Day 84 and was defined as no more than two prior dose adjustments and the serum tT4 concentration within the therapeutic range (1.0 to 5.4 μg/dL) when collected 4 to 6 hours post-tablet administration. After 84 ± 5 days of treatment, 87 of 107 evaluable cases (81.3%) were considered treatment successes. During the extended use phase of the study that allowed additional dose adjustments, 87 of 107 evaluable cases (81.3%) were considered treatment successes after 168 ± 5 days of treatment.
Clinical signs of hypothyroidism (lethargy, weight gain, hypercholesterolemia, bradycardia, cold intolerance, and dermatologic conditions such as alopecia, seborrhea, hyperpigmentation, myxedema, hyperkeratosis, scaling, and pyoderma) generally improved during the study.
ANIMAL SAFETY: A comprehensive literature review identified publications that reported clinical signs and adverse reactions resulting from oral and parenteral levothyroxine exposure in dogs.
Reported exposure to levothyroxine sodium, even at high-dose multiples, was well tolerated in the dogs included in the studies. Adverse reactions reported in naturally hypothyroid and euthyroid dogs exposed to levothyroxine sodium equivalent to 0.5X to 2X the initial 0.1 mg/10 lb (0.01 mg/lb, 0.022 mg/kg) twice daily ThyroKare dose were restlessness, lethargy, hyperactivity, anorexia, polyphagia, polyuria, polydipsia, periodic lateral recumbency, tachypnea, syncope, tachycardia, hyperthermia, pruritus, alopecia, skin scaling, dermatitis, otitis externa, change in coat color, weight loss, vomiting, borborygmus, diarrhea, epistaxis, leukocytosis, and elevated serum total thyroxine. Two studies of naturally hypothyroid dogs reported liver enzyme elevations [ALP, ALT, or aspartate aminotransferase (AST)] related to levothyroxine sodium administration that resolved in most dogs by 10 to 18 weeks after initiation of exposure. In one of the studies, seven dogs had a hematocrit and red blood cell count above the upper reference limit at the end of the study. In a study of euthyroid dogs, exposure to 0.5 mg/m2 levothyroxine sodium (approximately equivalent to the initial ThyroKare dose) for 8 weeks was associated with a decrease in pituitary thyrotrope volume density and morphologic changes consistent with thyroid gland inactivity. After cessation of treatment, the changes were reversible.
Chronic exposure to 25X the initial dose resulted in transient increases in bone metabolism, but bone turnover returned to near normal levels after two months of continuous exposure. Study dogs also exhibited transient increases in serum phosphorus and calcium, hyperthermia, tachycardia, and tachypnea. Additional adverse reactions reported in experimental studies that evaluated parenteral exposures to levothyroxine (approximately 2.5X to 25X the initial oral daily dose) included cardiovascular and dynamic conduction changes, anorexia, polycythemia, fine muscle tremors, and death.
STORAGE CONDITIONS: Store at 20°-25°C (68°-77°F) with excursions allowed between 15° and 30°C (59° and 86°F) and protect from light.
See bottom of bottle for Lot and Expiration Date.
How Supplied
ThyroKare (levothyroxine sodium tablets), USP is available as colored tablets in nine strengths: 0.1 mg-yellow; 0.2 mg-pink; 0.3 mg-green; 0.4 mg-light pink; 0.5 mg-white; 0.6 mg-dark blue-violet; 0.7 mg-pinkish orange; 0.8 mg-light blue; and 1.0 mg-tan, in bottles of 180 and 1,000 tablet counts.Approved by FDA under NADA # 141-539
References
[1]B. Chow and A. French, “Conversion of atrial fibrillation after levothyroxine in a dog with hypothyroidism and arterial thromboembolism,” J Small Anim Pract, vol. 55, no. 5, pp. 278-282, 2014.[2]J. A. Flood and J. P. Hoover, “Improvement in myocardial dysfunction in a hypothyroid dog,” Can Vet J, vol. 50, pp. 828-834, 2009.
[3]D. E. Phillips and K. R. Harkin, “Hypothyroidism and myocardial failure in two Great Danes,” J Am Anim Hosp Assoc, vol. 39, pp. 133-137, 2003.
[4]J. K. Sangster, D. L. Panciera and J. A. Abbott, “Cardiovascular effects of thyroid disease,” Compend Contin Educ Vet, vol. 35, no. 7, p. E1-E10, 2013.
[5]G. Le Traon, S. Burgaud and L. J.I. Horspool, “Pharmacokinetics of total thyroxine in dogs after administration of an oral solution of levothyroxine sodium,” J Vet Pharmacol Therap, vol. 31, pp. 95-101, 2008
Rev.12/20
Manufactured by: Neogen Corporation, 944 Nandino Blvd., Lexington, KY 40511
800.525.2022
NEOGEN.com
|
|
|
NDC: |
Item No. |
|
|
0.1 mg |
180 tablets |
59051-9100-0 |
09100 |
L7169-1220 |
|
0.1 mg |
1000 tablets |
59051-9100-8 |
09101 |
L7178-1220 |
|
0.2 mg |
180 tablets |
59051-9102-0 |
09102 |
L7170-1220 |
|
0.2 mg |
1000 tablets |
59051-9102-8 |
09103 |
L7179-1220 |
|
0.3 mg |
180 tablets |
59051-9104-0 |
09104 |
L7171-1220 |
|
0.3 mg |
1000 tablets |
59051-9104-8 |
09105 |
L7180-1220 |
|
0.4 mg |
180 tablets |
59051-9106-0 |
09106 |
L7172-1220 |
|
0.4 mg |
1000 tablets |
59051-9106-8 |
09107 |
L7181-1220 |
|
0.5 mg |
180 tablets |
59051-9108-0 |
09108 |
L7173-1220 |
|
0.5 mg |
1000 tablets |
59051-9108-8 |
09109 |
L7182-1220 |
|
0.6 mg |
180 tablets |
59051-9110-0 |
09110 |
L7174-1220 |
|
0.6 mg |
1000 tablets |
59051-9110-8 |
09111 |
L7183-1220 |
|
0.7 mg |
180 tablets |
59051-9112-0 |
09112 |
L7175-1220 |
|
0.7 mg |
1000 tablets |
59051-9112-8 |
09113 |
L7184-1220 |
|
0.8 mg |
180 tablets |
59051-9114-0 |
09114 |
L7176-1220 |
|
0.8 mg |
1000 tablets |
59051-9114-8 |
09115 |
L7185-1220 |
|
1.0 mg |
180 tablets |
59051-9126-0 |
09125 |
L7166-1220 |
|
1.0 mg |
1000 tablets |
59051-9126-8 |
09126 |
L7177-1220 |
CPN: 1491061.1
944 NANDINO BLVD, LEXINGTON, KY, 40511-1205
| Telephone: | 859-254-1221 | |
| Order Desk: | 800-525-2022 | |
| Fax: | 859-255-5532 | |
| Website: | http://animalsafety.neogen.com | |
| Email: | inform@neogen.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
