Synovex LA-F (Canada)
This treatment applies to the following species:trenbolone acetate and estradiol benzoate extended release implants
VETERINARY USE ONLY
For Feedlot Steers and Feedlot Heifers
DIN 02485133
Description
SYNOVEX® LA-F (trenbolone acetate and estradiol benzoate) is a growth promoting implant containing 200 mg of trenbolone acetate and 28 mg of estradiol benzoate with a porous polymer film coating that extends the pay-out period of the final formulation to over 200 days. Each implant consists of 8 pellets. Ten implants are provided in each cartridge.
Synovex LA-F Indications
To increase rate of weight gain and improve feed efficiency for up to 200 days in steers and heifers fed in confinement for slaughter. Product efficacy was confirmed in a growth-performance trial conducted in feedlot steers and heifers weighing between 235 and 350 kg at the time of implantation.
Note: Studies have demonstrated that the administration of SYNOVEX LA-F implants can result in decreased marbling scores when compared to non-implanted steers and heifers.
Dosage and Administration
Feedlot Cattle: One implant (eight pellets) is administered to each steer or heifer by subcutaneous implantation in the middle one-third of the ear.
The 8 pellets which make up one implant of SYNOVEX LA-F are contained in one channel of a ten-implant cartridge. The ten-implant cartridge of SYNOVEX LA-F is designed to be used exclusively with a SYNOVEX implanting device.
Directions For Use
Implant complete contents of one cartridge cell per steer or heifer. Approved implantation technique is fully described in the fold-out carton section.
Note: Never sacrifice careful, clean technique for speed of implantation.
HOW TO IMPLANT WITH SYNOVEX LA-F PELLETS
For use with the applicator.
Study the following instructions carefully, then proceed step by step, until the technique becomes routine. Many head can be implanted per hour by an experienced team, one member of which should be assigned to do nothing but the implantation. Care should be taken to insure the hands of the person administering the implant are clean and only sanitary instruments are used.
Step 1 Loading The Applicator
Load the applicator following the directions outlined in the instruction manual accompanying each applicator.
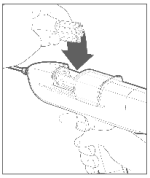
Step 2 Restraint
The animal must be confined in a restraint mechanism (squeeze chute or head gate). The implant site on the back of the ear should be prepared by scrubbing with a generous-sized piece of cotton that has been soaked in a germicidal solution.
Note: If implanting horned cattle, greater safety is provided when the head is controlled by the use of a bull lead (nose tongs).
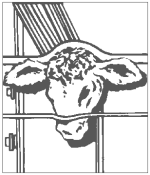
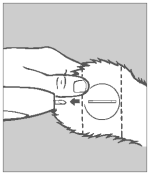
Step 3 Implant Site
Divide the ear into three imaginary sections as illustrated. The implanted pellets should be deposited in the center one-third of the ear as shown. To accomplish this, the applicator gun needle should be inserted in the outer one-third of the ear as indicated by the “X” in the illustration. Implanting too close to the head may cause abnormal sexual behavior. Care should be taken to avoid severing the major arteries of the ear.
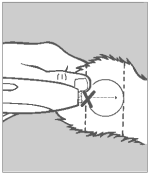
Step 4 Insert Needle
Grasp the ear with one hand. Holding the applicator firmly with the other hand, penetrate the skin at the point shown by the “X”. Thrust the needle under the skin taking care not to penetrate the cartilage. Ease the applicator forward (toward the base of the ear) until the full needle length is beneath the skin.
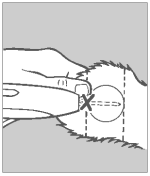
Step 5 Pellet Implantation
When the needle is completely inserted, activate the instrument by squeezing the trigger completely. Do not withdraw the needle, but allow the automatic needle retractor to release the pellets. This will allow the pellets to be deposited in a straight line in the path of the retracted needle.
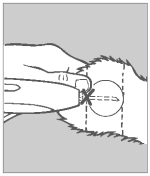
Step 6 Inspection
Check the implant site. If properly administered, the implants should lie in a straight line under the skin.
Re-cock the gun by grasping the barrel with your left hand and lifting the cocking bolt with your right hand. Fully retract the bolt to the rear of the device. Then, return the bolt forward to its locked position. Disinfect the applicator needle in a germicidal solution. The applicator gun is now ready to implant the next animal.
CAUTIONS:
For use in feedlot steers and feedlot heifers only. Do not use in heifers intended for subsequent breeding.
Warnings
No withdrawal period is required when treated according to the label.
Do not use in calves to be processed for veal.
Do not use in dairy cattle.
Implant pellets in ear only. Do not attempt to salvage the implanted site for human or animal food.
Keep out of reach of children.
Adverse Reactions
Although all adverse reactions are not reported, the following information is based on voluntary post-approval drug experience reporting. It is generally recognized that this results in significant under-reporting. The adverse events listed here reflect reporting and not necessarily causality. Bulling or riding behavior has been reported rarely with use of this product. Vaginal and rectal prolapse, udder development, ventral edema and elevated tailheads have also been reported.
Storage
Store unopened product between 20 and 25°C with excursions between 15 and 30°C. Avoid excessive heat and humidity. Once the pouch is opened, unused product may be stored in the end-folded pouch (away from light) for up to six months at 2-8°C or at 15-30°C for up to one month.
Zoetis® and Synovex are registered trademarks of Zoetis or its licensors.
Zoetis Canada Inc., Kirkland QC H9H 4M7
|
NET 10 cartridges of 10 doses (100 implants) |
40043447 10022441-14-1 |
CPN: 1198564.1
16,740 TRANS-CANADA HIGHWAY, KIRKLAND, QC, H9H 4M7
| Order Desk: | 800-663-8888 | |
| Technical Services Canada: | 800-461-0917 | |
| Technical Services USA: | 800-366-5288 | |
| Website: | www.zoetis.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
