Syncaine
This treatment applies to the following species:(tricaine methanesulfonate)
KEEP TIGHTLY CLOSED
USE ONLY FRESH SOLUTION
Before using this drug, read package insert for complete product information.
Store at room temperature (Approximately 25°C (77°F))
KEEP OUT OF THE REACH OF CHILDREN
SYNCAINE is intended for the temporary immobilization of fish, amphibians, and other aquatic, cold-blooded animals (poikilotherms) as an aid in handling during manual spawning (fish stripping), weighing, measuring, marking, surgical operations, transport, photography, and research.
Warnings
Do not use within 21 days of harvesting fish for food. Use in fish intended for food should be restricted to Ictaluridae, Salmonidae, Esocidae, and Percidae, and water temperature exceeding 10°C (50°F). In other fish and in cold-blooded animals, SYNCAINE should be limited to hatchery or laboratory use. Avoid inhaling or getting into eyes.
Chemistry
SYNCAINE is the methanesulfonate of meta-amino benzoic acid ethylester, or simply ethyl m-amino benzoate. It is thus an isomer of benzocaine having the formula C9H11O2N + CH3SO3H and the following structure:
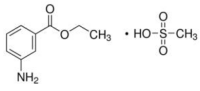
SYNCAINE is a fine white crystalline powder. Its molecular weight is 261.3. Soluble to 11%, it forms clear, colorless, acid solutions in water.
Toxicology
Comparative toxicologic studies carried out on fish and frogs gave the following results:
FISH TOXICITY STUDIES - The toxicity of tricaine methanesulfonate was measured by standard methods in laboratory bioassays with rainbow trout, brown trout, brook trout, lake trout, northern pike, channel catfish, bluegill, largemouth bass, and walleye. The 24, 48 and 96 hour LC50 (lethal concentration for 50 percent of the animals) values for trout ranged from 52 to 31 mg/liter; for northern pike, from 56 to 48 mg/liter; for catfish, from 66 to 50 mg/liter; for bluegill and largemouth bass, from 61 to 39 mg/liter; and for walleye, the values were 49 to 46 mg/liter.
Safety index: The safety indices for tricaine methanesulfonate refer to the margin between concentrations which cause anesthesia and mortality. They are expressed by the quotient of the lethal concentration for 50 percent of the fish (LC50) and the effective concentration for 50 percent of the fish (EC50).
Safety Indices for Rainbow Trout and Channel Catfish at 12°C (54°F).
|
Species |
Exposure (min.) |
LC50 (mg/liter) |
EC50 (mg/liter) |
Index |
|
Rainbow trout1 |
15 |
65 |
32 |
2.0 |
|
“ |
30 |
57 |
32 |
1.8 |
|
“ |
60 |
56 |
29 |
1.9 |
|
Channel catfish2 |
15 |
139 |
47 |
3.0 |
|
“ |
30 |
118 |
45 |
2.6 |
|
“ |
60 |
110 |
46 |
2.4 |
FROG TOXICITY STUDIES3 - Frogs were put into various concentrations of tricaine methanesulfonate for 30 minutes and then transferred to tap water in order to determine the LC50. The LC50 was 6.2 percent tricaine methanesulfonate. Therefore, the anesthetic must be used in very high concentration before it is fatal to frogs.
I. Directions For Use On Fish
Concentrations
SYNCAINE is effective and safe for the anesthesia of fish when used as directed. Its use is governed by, and can be tailored to, the needs of individual fishery personnel. Sedation and various rates of anesthetization are controlled by the concentration. The versatility of SYNCAINE is demonstrated by the fact that it has been used in fisheries at levels ranging from 10 to 1,000 mg/liter3. The action of the anesthetic is slowed at cooler temperatures, in extremely soft water (approximately 10 mg/liter of CACO3, or less), and in larger fish4. Also, efficacy may vary with species4. Thus, it is imperative that preliminary tests of anesthetic solutions be made against small numbers of fish to determine the desired rate of anesthesia and exposure times for the specific lots of fish under prevailing conditions.
The following tables may be used as guidelines in selecting concentrations of SYNCAINE for the anesthetization of various fishes:
Table 1 - Concentrations Required For Rapid Anesthesia
(Induction time less than 2-5 minutes; used in spawning, marking, measuring, and some surgical operations)
|
Fish |
Temperature |
Concentration (mg/liter) |
Max. tolerated exposure time* (min.) |
Recovery time in fresh water (min.) |
|
Salmonidae 4 |
7 - 17°C |
80 - 135 |
4 - 12 |
3 - 19 |
|
(Pacific and Atlantic salmon; trout; chars; etc.) |
||||
|
Esocidae 5 |
8 - 12°C (46 - 54°F) |
150 |
8 - 28 |
8 - 31 |
|
(Northern Pike; muskellunge) |
||||
|
Cyprinidae 3 |
16°C |
150 - 200 |
|
|
|
(Carp; goldfish) |
||||
|
Ictaluridae 2 |
7 - 27°C |
140 - 270 |
4 - 11 |
3 - 24 |
|
(Channel catfish) |
||||
|
Centrarchidae 4 |
10 - 27°C |
260 - 330 |
3 - 5 |
7 - 11 |
|
(Bluegill; largemouth bass) |
||||
|
Percidae3 |
10 - 16°C |
100 - 120 |
7 - 18 |
5 - 40 |
|
(Walleye) |
||||
|
Pet and Tropical1 |
||||
|
Live-bearers |
24 - 27°C |
85 |
12 hrs. |
|
|
Egg layers |
24 - 27°C |
75 |
12 hrs. |
|
*Maximum tolerated exposure time (in minutes) of fish to SYNCAINE solution.
Table 2 - Concentrations Required For Moderately Rapid Anesthesia
(Induction time less than 15-20 minutes; used in surgical operations and in spawning and marking where longer exposures are more important than rapid immobilization)
|
Fish |
Temperature |
Concentration (mg/liter) |
Maximum tolerated exposure time* (min.) |
Recovery time in fresh water (min.) |
|
Salmonidae 4 |
7 - 17°C |
50 - 60 |
30 or > |
2 - 20 |
|
(Pacific and Atlantic salmon; trout; chars; etc.) |
||||
|
Ictaluridae 2 |
7 - 27°C (45 - 81°F) |
70 |
30 or > |
1 - 10 |
|
(Channel catfish) |
||||
*Maximum tolerated exposure time (in minutes) of fish to SYNCAINE solution.
Table 3 - Concentrations Required For Sedation
(Induction within 15 minutes; used in fish transport)
|
Fish |
Temperature |
Concentration (mg/liter) |
Maintenance of sedation (hr.) |
|
Salmonidae 4 |
7 - 17°C |
15 - 30 |
6 |
|
(Pacific and Atlantic salmon; trout; chars; etc.) |
|||
|
Esocidae 5 |
|
40 |
|
|
(Chain pickerel) |
|||
|
Ictaluridae 2 |
7 - 27°C |
20 - 40 |
6 |
|
(Channel catfish) |
|||
|
Centrarchidae 8 |
|
25 |
8 - 13 |
|
(Bluegills) |
|||
|
Pet and Tropical1 |
|||
|
[Bettas, Piranhas, etc. (uncrowded) |
24 - 27°C |
66 |
48 |
|
Goldfish] |
24 - 27°C |
37 |
48 |
IMPORTANT: Since, in many cases, relatively rapid rates of anesthesia can be achieved only by exceeding the lethal concentration of SYNCAINE, it is necessary to return anesthetized fish to fresh water before they are overexposed. Excessive exposures are avoided by observing the following sensory and motor responses of the fish which characterize progressively deeper levels of anesthesia:
Sedation - Decreased reactivity to visual and vibrational stimuli; opercular activity reduced.
Total loss of equilibrium - Fish turns over; locomotion ceases; fish swims or extends fins in response to pressure on caudal fin or peduncle.
Total loss of reflex - No response to pressure on caudal fin or peduncle; opercular rate slow and erratic.
Medullary collapse - Opercular activity ceases.
Laboratory and field investigations3,9 have shown that the action of SYNCAINE is readily reversed when the fish are transferred to fresh water before opercular activity ceases. Additional exposure following medullary collapse may result in mortality. A rough estimate of the safe total exposure can be made by multiplying the time required for anesthesia by a factor of 2 or 3.
Water
Since SYNCAINE is very soluble (1:9) in water, it dissolves with equal readiness in spring water, tap water, or seawater. Do not use distilled or deionized water, or water containing chlorine, heavy metals (copper, zinc, etc.), or other toxic contaminants. The anesthetic solution should be well oxygenated, and its temperature should be similar to that of the water from which the fish are taken. In the field, many water quality problems are eliminated by using natural water to which the fish are acclimated, provided the water does not possess high chemical or biologic oxygen demand.
METHODS OF APPLICATION
1. General anesthesia: For most situations where rapid or moderately rapid anesthesia is required, SYNCAINE may be applied in a bath, i.e., the fish are immersed in the anesthetic solution. Containers may be of glass, plastic, steel, aluminum, or other suitable material. However, do not use galvanized or brass containers unless treated or sealed to prevent dissolution of zinc. Size of container is determined by individual needs, but the fish should not be overcrowded. Discard anesthetic solutions when a loss in potency is noted, or when the solutions become fouled with mucus or excrement.
2. For surgery and certain physiologic studies, the fish may be anesthetized to loss of reflex, removed from the anesthetic, and then positioned so that the gills are bathed in a sedating concentration of SYNCAINE. Some investigators have developed flowing, re-circulating systems for bathing the gills with anesthetic during surgery.
Large fishes such as sharks and rays are anesthetized within minutes by spraying the gills with a 1 g/liter solution of tricaine methanesulfonate.10 The application is made by means of a water pistol, bulb syringe, hand pump, etc.
3. Transport - SYNCAINE has been used to sedate fish during transport. It is more successful in cold than in warm water, and it is instrumental in reducing injuries because of hyperactivity. Fish are usually transported by means of distribution units (tank trucks), or by air in plastic bags.11,12 In either case, the fish should be fasted before-hand to reduce metabolic wastes. Also, some workers suggest pre-transport sedation for several hours to lower metabolism. With distribution units, the fish may be fasted and sedated prior to loading. The anesthetic solution is prepared in the distribution unit and oxygenated. Then, the fish are added and temperature acclimated.
In air shipments, the anesthetic solution is placed in a suitable plastic bag, the sedated fish are added, the bag inflated with oxygen, tied securely, and placed in a second bag. This bag is also tied, and then placed on ice in an insulated container13. A modification of this method involves complete anesthesia of the fish, and placing them in water bags which contain no anesthetic. In any case, upon arrival, the fish should be acclimated slowly to new environmental temperatures.
Preparation Of Syncaine Solutions
Prior to use, SYNCAINE may be weighed out into amounts which are convenient for the volume of water to be used. A handy unit is 2 g. since this quantity in 5 gallons of water yields a concentration of about 100 mg/liter. For rough approximations, one level teaspoonful contains 2.0 to 2.5 g. Thus, a level teaspoonful of anesthetic in 5 gallons gives a concentration of about 120 mg/liter.
To convert mg/liter into g/gal.: multiply number of mg. by 0.00378.
e.g. 80 mg/liter = 80 x 0.00378 = 0.302 g/gal.
To convert mg/liter into a ratio of SYNCAINE to water: divide 1,000,000 by the number of mg.
e.g. 80 mg/liter = 1,000,000 ÷ 80 = 1:12,500
Limitations In Use
Since SYNCAINE is taken up into the blood of fish, residues of the drug may occur in edible tissues. However, the residues dissipate rapidly after the fish are placed in fresh water14. Do not use within 21 days of harvesting fish for food. Use in fish intended for food should be restricted to Ictaluridae, Salmonidae, Esocidae, and Percidae, and water temperature exceeding 10°C (50°F).
Withdrawal in fresh water is unnecessary for non-food fishes such as goldfish, bait fish, and ornamentals. Also, withdrawal is unnecessary for sublegal sizes of the following species of fish because they are not used as food immediately following anesthesia (Table 4).
Table 4 - Sublegal Sizes of Fish Species Not Used as Food Immediately after Anesthesia15
|
Species |
Size (in.) |
|
Pink salmon |
6 |
|
Chum salmon |
6 |
|
Coho salmon |
6 |
|
Sockeye salmon |
6 |
|
Chinook salmon |
6 |
|
Cutthroat trout |
6 |
|
Steelhead trout |
8 |
|
Rainbow trout |
6 |
|
Atlantic salmon |
10 |
|
Brown trout |
6 |
|
Brook trout |
6 |
|
Lake trout |
5 |
|
Splake trout |
6 |
|
Grayling |
6 |
|
Northern pike |
12 |
|
Muskellunge |
12 |
|
Channel catfish |
6 |
|
Flathead catfish |
6 |
|
Bluegill |
3 |
|
Redear sunfish |
3 |
|
Smallmouth bass |
5 |
|
Largemouth bass |
5 |
|
Walleye |
6 |
PRECAUTIONS
1. Avoid inhaling SYNCAINE or getting it into the eyes.
2. Always conduct preliminary tests with SYNCAINE to determine desired rates of anesthesia and optimal length of exposure.
3. Do not overexpose fish to lethal levels of SYNCAINE.
4. Do not anesthetize more fish than can be handled effectively.
5. Do not contaminate eggs or sperm with SYNCAINE when stripping fish.
6. Do not use water containing chlorine, or other toxic agents.
7. Ensure adequate oxygen in anesthetic solution.
8. Discard anesthetic solutions when fouled with mucus or metabolic wastes.
9. Do not discard SYNCAINE solutions into water supplies or natural waters.
10. Store SYNCAINE solutions in a cool place away from light.*
11. Discard stock solutions of SYNCAINE after several days.*
12. Treated fish destined for food must be held in fresh water above 10°C (50°F) for 21 days before use.
* The color of SYNCAINE solutions may change rapidly to yellow or brown when exposed to light. This does not affect activity in any significant way. However, for best results, use freshly prepared solutions. A 10 percent solution stored at room temperature shows no significant loss of potency after three days, but after 10 days, a brownish color and an activity decrease of 5 percent is observed.
Ii. Guidelines For Use On Amphibians:
Table 5 - Effects of Varying Concentrations of SYNCAINE on Salamanders
|
Salamander |
Concentration* |
Duration of Anesthesia* |
Remarks |
|
EMBRYOS |
1:10,0003b |
2 days |
No adverse effects |
|
Ambystoma opacum |
1:3,0003c |
to 30 min. |
|
|
LARVAE |
1:10,0003b |
2 days |
No adverse effects |
|
1:12,0003f |
10 - 15 min. |
||
|
1:20,0003f |
10 - 15 min. |
||
|
Ambystoma opacum |
1:3,0003c |
to 30 min. |
No adverse effects |
|
ADULTS |
1:1,0003b |
few min. |
No adverse effects |
|
1:3,0003b |
3 days |
||
|
Newts |
1:1,0003b |
few min. |
No adverse effects |
|
1:10,0003b |
2 days |
||
|
Triturus sp. |
1:1,0003k |
20 min. |
No adverse effects |
|
Triturus viridescens |
1:3,0003g |
1 hour |
|
|
Mole salamanders |
|||
|
Ambystoma opacum |
1:3,0003c |
to 30 min. |
No adverse effects |
|
Ambystoma tigrinum |
1:2,0003j |
15 - 30 min. |
|
|
Ambystoma punctatum |
1:2,0003j |
15 - 30 min. |
|
|
Mudpuppy |
|||
|
Necturus maculosus |
1:1,5003i |
to 6 hours |
** |
* When an individual of any of the species listed is exposed at the designated concentration, the data available suggests that the animal may be safely maintained under anesthesia for the time noted. Prolonging exposure to the anesthetic beyond the time indicated may cause deaths. See PRECAUTIONS.
** Maintenance dose, 0.1 of induction concentration. At exposure to induction concentration for more than 20-30 minutes, renal circulation becomes sluggish or stops.
Table 6 - Effects of Varying Concentrations of SYNCAINE on Frogs
|
Frog |
Concentration* |
Duration of Anesthesia* |
Remarks |
|
EMBRYOS |
1:1,0003b |
few min |
No adverse effects. |
|
1:10,0003b |
2 days |
||
|
1:15,0003h |
3 days |
||
|
TADPOLES |
1:1,0003j |
30 min. |
No adverse effects. |
|
1:3,0003f |
10 - 15 min. |
||
|
1:10,0003b |
2 days |
||
|
1:15,0003h |
3 days |
||
|
Rana sp. |
1:5,0003k |
5 hours |
No adverse effects. |
|
Rana pipiens |
1:1,0003j |
15 - 30 min. |
|
|
1:3,3333a |
2 min. |
||
|
variable3d |
1 hour |
||
|
ADULTS |
1:1,0003e |
30 min. |
No adverse effects. |
|
Leopard frog |
|||
|
Rana pipiens |
1:3,0003c |
to 30 min. |
No adverse effects. |
|
Eastern wood frog |
|||
|
Rana sylvatica |
1:8,0003l |
5 - 10 min. |
Only slightly under anesthesia |
* When an individual of any of the species listed is exposed at the designated concentration, the data available suggests that the animal may be safely maintained under anesthesia for the time noted.
Prolonging exposure to the anesthetic beyond the time indicated may cause deaths. See PRECAUTIONS.
AVAILABILITY OF SYNCAINE
Bottles of 1 kilogram, 100 grams, and 10 grams.
REFERENCES
1. Marking, L.L.: Investigations in Fish Control. 12. Toxicity of MS-222 to Selected Fishes, U.S. Bureau of Sport Fisheries and Wildlife, Resource Publication 18, 1966.
2. Schoettger, R.A., Walker, C.R., Marking, L.L., and Julin, A.M.: MS-222 as an Anesthetic for Channel Catfish; its Toxicity, Efficacy, and Muscle Residues, U.S. Bureau of Sport Fisheries and Wildlife, Resource Publication 33, 1967.
3. Personal communications:
a. Bernheimer, W.M., New York University College of Medicine, New York, N.Y.
b. Butler, E.G., Princeton University, Dept. of Biology, Princeton, N.J.
c. Dalton, H.D., and Charipper, H.A., Washington Square College, Dept. of Biology New York, N.Y.
d. Etkin, W., City College, Dept. of Biology, New York, N.Y.
e. Goss, R.J., Brown University, Providence, R.I.
f. Kollros, J.J., State University, Iowa City, Iowa.
g. Manner, H.W.: Anaesthetize those planaria, Turtox News 35:135, 1957.
h. Rose, S.M., University of Illinois, Urbana, Ill.
i. Schatzmann, J.H., Harvard Medical School, Boston, Mass.
j. Taylor, A.C., Rockefeller Institute of Medical Research, New York, N.Y.
k. Thornton, C.S., Kenyon College, Dept. of Biology, Gambier, Ohio.
l. Van Stone, J.M., Trinity College, Dept. of Biology, Hartford, Conn, Cited in Bove, FJ.,: MS-222 Sandoz-the anesthetic of choice for fish and other cold-blooded organisms, Sandoz News, no.3, 12p., 1962.
4. Schoettger, R.A., and Julin, A.M.: Investigations in Fish Control: 13. Efficacy of MS-222 as an Anesthetic on Four Salmonids, U.S. Bureau of Sport Fisheries and Wildlife, Resource Publication 19, 1966.
5. Schoettger, R.A.: Efficacy of MS-222 as an Anesthetic for Northern Pike, Muskellunge and Walleye, U.S. Bureau of Sport Fisheries and Wildlife.
6. Knight, A.E.: Intracellular hemoglobin crystallization in two centrarchids, the large-mouth bass and the bluegill, Progressive Fish Culturist 26:115 (no. 3) 1964.
7. Lumb, W.V. Anesthesia of Laboratory and Zoo Animals, in: Small Animal Anesthesia, Philadelphia, Lea and Febiger, 1963, pp. 269-310.
8. Webb, R.T., Distribution of bluegill treated with tricaine methanesulfonate (MS-222), Progressive Fish-Culturist 20:69 (no. 2) 1958.
9. Klontz, G.W.: Anesthesia of fishes, Proceedings of the Symposium on Experimental Animal Anesthesiology, Brooks Air Force Base, Dec. 14-16, 13p., 1964.
10. Gilbert, P.W., and Wood, F.G.: Methods of anesthetizing large sharks and rays safely and rapidly. Science 126: 212, 1957.
11. Mann, H., and Rajbanshi, K.G.: Anesthetic and Tranquilizer for Fish, Frogs and other Cold-blooded Organisms, Sandoz Bulletin No. 3350/182 e. Basle, Switzerland.
12. Tuumanen, P.: Experiments with MS-222 Sandoz in the Shipment of Live Trout in Plastic Pouches, Kalataloudellisen tukimostoimiston, Tiedomantoja, no. 2, 1966.
13. Lemarque, P.: Anesthesie et transport, Bull, Inf. Cons. Sup. Pêche 55:5, 1964.
14. Walker, C.R., and Schoettger, R.A.: Investigations in Fish Control: 15. Residues of MS-222 in Four Salmonids Following Anesthesia, U.S. Bureau of Sport Fisheries and Wildlife, Resource Publication 21, 1966.
15. Correspondence: Bureau of Fisheries, U.S. Department of Interior, 1968.
Manufactured by:
Syndel, 1441 W. Smith Road Ferndale, WA 98248
(800) 283-5292
(360) 384-5898
FAX (360) 384-0270
www.syndel.com
Approved by FDA under ANADA # 200-226
|
10 Grams |
ver. 011222 |
|
100 Grams |
ver. 010622 |
|
1 Kilogram |
ver. 010322 |
CPN: 1021005.3
Syndel USA
1441 W. SMITH ROAD, FERNDALE, WA, 98248
| Telephone: | 360-384-5898 | |
| Toll-Free: | 800-283-5292 | |
| Customer Service: | 360-384-5898 | |
| Order Desk: | 800-283-5292 | |
| Fax: | 360-384-0270 | |
| Website: | www.syndel.com | |
| Email: | info@syndel.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
