Percorten-V Injectable (Canada)
This treatment applies to the following species: Company: Elanco
Company: Elanco
PRESCRIBING INFORMATION
Desoxycorticosterone pivalate (DOCP) sterile suspension
DIN 02139227
Description
Percorten-V (Desoxycorticosterone pivalate [DOCP]) is a mineralocorticoid hormone and an analog of desoxycorticosterone. It is white, odourless and stable in air. It is practically insoluble in water, sparingly soluble in acetone, slightly soluble in methanol, ether and vegetable oils.
ACTIONS:
The chief effects of Percorten-V are on the metabolism of sodium, potassium and water. When the drug is given to the adrenalectomized animal, there is decreased excretion of sodium accompanied by increased excretion of potassium; the concentration of sodium in the blood is thereby increased whereas that of potassium is decreased. There is a concomitant increase in the volume of blood and extracellular fluids, with a fall in hematocrit. Percorten-V has very little, if any, effect on carbohydrate metabolism and none on the number of circulating lymphocytes or eosinophils.
CHEMISTRY:
The molecular weight is 414.58. It is designated chemically as 21-hydroxypregn-4-ene-3,20-dione pivalate. The empirical formula is C26H38O4 and the structural formula is:
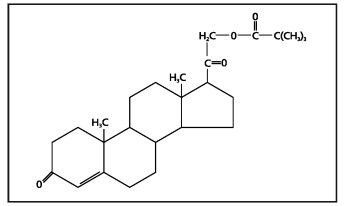
Percorten-V is a white mobile suspension; each mL contains 25 mg desoxycorticosterone pivalate. Inactive ingredients are water, methylcellulose, sodium carboxymethylcellulose, polysorbate 80, sodium chloride and thimerosal.
INDICATION:
For use in dogs as partial mineralocorticoid replacement therapy in cases of adrenocortical insufficiency.
Clinical Pharmacology
Desoxycorticosterone pivalate, like other adrenocorticoid hormones, is thought to act by controlling the rate of synthesis of proteins. It reacts with receptor proteins in the cytoplasm to form a steroid-receptor complex. This complex moves into the nucleus, where it binds to chromatin which results in genetic transcription of cellular DNA to messenger RNA. The steroid hormones appear to induce transcription and synthesis of specific proteins, which produce the physiological effects seen after administration.
Percorten-V (DOCP) is a long acting analog of desoxycorticosterone acetate, which is recognized as having the same qualitative effects as the natural mineralocorticoid aldosterone. It has very little, if any, effect on carbohydrate metabolism.
The most important effect of DOCP is to increase the rate of renal tubular absorption of sodium. This effect is seen most intensely in the thick portion of the ascending limb of the loop of Henle. It also increases sodium absorption in the proximal convoluted tubule, but this effect is less important in total sodium retention. Chloride ions follow the sodium ions out of the renal tubule.
Another important effect of DOCP is enhanced renal excretion of potassium. This effect is driven by the resorption of sodium ions which pulls potassium ions from the extracellular fluid into the renal tubules, thus promoting potassium excretion.
DOCP also acts to increase extracellular fluid volume. The enhanced retention of sodium, chloride and bicarbonate, creates an osmotic gradient that promotes water absorption from the renal tubules.
The extracellular fluid volume is supported, which expands blood volume and improves venous return to the heart and cardiac output. The expanded blood volume and increased cardiac output may result in elevated blood pressure. DOCP prevents the life threatening hypotensive shock and pre-renal azotemia observed in animals suffering from hypoadrenocorticism.
The effects of DOCP on electrolytes and extracellular fluid volume is dependent on a functioning kidney. Animals suffering from hypovolemia, pre-renal azotemia, and inadequate tissue perfusion must be rehydrated with intravenous fluid (saline) therapy before starting DOCP therapy. Primary renal disease should be ruled out before starting DOCP therapy. DOCP is an insoluble ester of desoxycorticosterone, the crystals are injected intramuscularly as a microcrystalline depot where they slowly dissolve over time.
DOSAGE:
DOSAGE REQUIREMENTS ARE VARIABLE AND MUST BE INDIVIDUALIZED ON THE BASIS OF THE RESPONSE OF THE PATIENT TO THERAPY. Begin treatment at a dose of 1.0 mg per pound of body weight every 25 days. In some patients the dose may be reduced. Serum sodium and potassium levels should be monitored to assure the animal is properly compensated. Most patients are well controlled with a dosage of 0.5 to 1.0 mg per pound of body weight, given every 21 to 30 days.
In treating canine hypoadrenocorticism, Percorten-V (DOCP) replaces the mineralocorticoid hormones only. Glucocorticoid replacement must be supplied by small doses of glucocorticoid hormones (e.g. prednisone or prednisolone). Oral supplementation with salt (NaCl) is not necessary with animals receiving DOCP.
ADMINISTRATION:
Before injection, shake the vial thoroughly to mix the microcrystals with the suspension vehicle. Percorten-V (DOCP) sterile suspension is to be injected intramuscularly. Care should be used to prevent inadvertent intravenous injection, which may have adverse effects on the animal.
Contraindications
The safe use of this drug in pregnant dogs has not been established. Do not use in dogs suffering from congestive heart disease or edema.
Warnings
Keep this and all drugs out of the reach of children. In case of human consumption, contact a physician or Poison Control centre immediately.
Adverse Reactions
Some patients are more sensitive to the actions of Percorten-V (DOCP) and may exhibit side effects to an exaggerated degree. Some patients may show signs of hypernatremia or hypokalemia. The dosage of DOCP should be reduced in these patients.
Like other adrenocortical hormones, DOCP may cause side effects if dosage is too high or prolonged. It may cause polyuria, polydipsia, increased blood volume, edema and cardiac enlargement. Excessive weight gain may indicate fluid retention secondary to sodium retention. Percorten-V should be used with caution in patients with congestive heart disease, edema or renal disease.
On rare occasions, irritation at the site of injection has been reported.
Storage
Store at room temperature (15-30°C). Protect from light.
Avoid freezing.
AVAILABILITY: 4 ML MULTI-DOSE VIALS:
Each mL of sterile aqueous suspension contains 25 mg desoxycorticosterone pivalate (DOCP), 10.5 mg methylcellulose, 3 mg sodium carboxymethylcellulose, 1 mg polysorbate 80, 8 mg sodium chloride with 0.002% thimerosal as preservative.
Elanco Canada Limited, 1919 Minnesota Court, Suite 401, Mississauga, Ontario L5N 0C9
Percorten, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
03May2022
CPN: 1231022.5
1919 MINNESOTA COURT, SUITE 401, MISSISSAUGA, ON, L5N 0C9
| Customer Service: | 800-265-5475 | |
| Fax: | 519-821-7831 | |
| Website: | www.elanco.ca | |
| Email: | elancocanadacustomerservice@elancoah.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
