Naquasone Injectable Solution (Canada)
This treatment applies to the following species:Trichlormethiazide (USP, 10 mg/mL)/Dexamethasone Acetate (USP, 0.55 mg/mL) Solution
(equine) (bovine)
Sterile
Diuretic Glucocorticoid
DIN 00555673
VETERINARY USE ONLY
Structural Formula And Chemistry
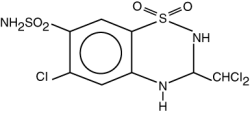
Trichlormethiazide (USP)
Molecular Formula: C8H8Cl3N3O4S2
Molecular Weight: 380.65
Chemical Name: 2H-1, 2, 4-Benzothiadiazine-7-sulfonamide, 6-chloro-3-(dichloromethyl)-3-, 4-dihydro-, 1.1-dioxide.
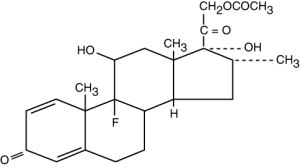
Dexamethasone Acetate USP
Molecular Formula: C24H31FO6•H2O
Molecular Weight: 434.50
Chemical Name: Pregna-1, 4-diene-3, 20-dione, 21-(acetyloxy)-9-fluoro-11, 17-dihydroxy-16-methyl-, (11β, 16α)-monohydrate.
Description
NAQUASONE injectable solution contains trichlormethiazide USP, and dexamethasone acetate USP. It combines the effective diuretic activity of trichlormethiazide with the specific anti-inflammatory activity of dexamethasone for complementary action in the reduction of physiological inflammatory edematous conditions of the equine, and in the reduction of physiological parturient edema of the mammary gland and associated structures of cattle.
Composition
Each ml contains trichlormethiazide USP 10 mg, dexamethasone acetate USP 0.55 mg as active ingredients and benzyl alcohol 26.14 mg as a preservative.ACTION: The mechanism of action by which trichlormethiazide affects diuresis in edematous conditions is by an inhibitory effect on the reabsorption of sodium and chloride in the renal tubules, thereby enhancing excretion of sodium, chloride and water, while apparently having a considerably lessened and more temporary effect on the excretion of potassium and bicarbonate.
The corticoid dexamethasone possesses anti-inflammatory activity. Clinical experience shows that edema is greatly reduced following parenteral treatment with 20 ml of NAQUASONE injectable solution. Effective doses do not cause sodium and fluid retention or potassium loss when used as recommended, but rather enhance the action of the diuretic in NAQUASONE injectable solution by the specific anti-inflammatory effect exerted by corticosteroids.
INDICATIONS: Equine: NAQUASONE injectable solution is indicated for the treatment of inflammatory conditions of the equine. Bovine: NAQUASONE injectable solution is indicated for the treatment of physiologic parturient edema of the mammary gland and associated structures of cattle.
DOSAGE AND ADMINISTRATION: Equine: 0.5 mI/45 kg (100 lb) of body weight intramuscularly twice the first day, then followed by 0.5 mI/45 kg (100 lb) of body weight once daily for two additional days. The extent and duration of treatment will depend upon the severity of the condition and response to medication. Treatment must be administered for at least two days. Bovine: Prior to, at the time of, or immediately following parturition inject 20 ml (200 mg trichlormethiazide, 10 mg dexamethasone) intramuscularly, and give one bolus (200 mg trichlormethiazide, 5 mg dexamethasone) orally 12 and 36 hours later.
The usual case of edema seen under field conditions will generally begin to respond within 24-48 hours after NAQUASONE injectable solution therapy is initiated. Most cases will subside in three to four (3-4) days. If no results are evident in three (3) days, it is recommended that the animal be re-examined.
CAUTIONS: Trichiormethiazide is a potent, benzothiadiazide diuretic agent required in low milligram doses for saluretic effectiveness and should be used accordingly. Electrolyte depletion effects may be seen with over-vigorous therapy and maximum doses should not be used over an extended period of time. Potassium supplements have not generally been necessary with trichlormethiazide and their administration routinely should not be required. Hypochloremia is unlikely with trichlormethiazide at recommended doses. Animals with severe renal function impairments should be checked for hyperkalemia and acidosis, since therapy with benzothiadiazine diuretics like trichlormethiazide may be contraindicated in these patients.
It must always be kept in mind that dexamethasone is an extremely potent drug with profound physiologic effect. All of the precautions and contraindications for adrenocortical hormones must be followed. Animals receiving the drug should be under close observation.
Administration of corticosteroid-containing products such as NAQUASONE injectable solution may cause premature parturition with subsequent retained placenta. Metritis is also an occasional sequel.
NAQUASONE injectable solution may be administered to animals with acute or chronic bacterial infections provided the infections are controlled with appropriate antibiotic or chemotherapeutic agents. It should be kept in mind that dexamethasone like cortisone, through its anti-inflammatory action, may mask the signs of infection such as elevation of temperature. For this reason, if any questionable findings occur, it may be necessary to temporarily discontinue NAQUASONE injectable solution until a diagnosis is made. Proper antibacterial therapy may then be instituted together with NAQUASONE injectable solution. The possible action of dexamethasone in delaying wound healing should be kept in mind. Clinical studies with NAQUASONE injectable solution in the equine did not suggest any adverse effect.
Do not administer to pregnant mares.
Warnings
Cattle: Treated cattle must not be slaughtered for use in food for at least 21 days after the latest treatment with this drug. Milk taken from treated cattle during treatment and within 48 hours after the latest treatment must not be used as food. Horses: This drug is not to be administered to horses that are to be slaughtered for use in food. Keep out of reach of children.Clinical Pharmacology
Trichlormethiazide is an effective diuretic agent of the benzothiadiazine series. Studies in man and experimental animals show that trichlormethiazide causes a favourable pattern of less potassium excretion than chlorothiazide or hydrochlorothiazide. The clinically determined saluretic potency of trichlormethiazide is estimated to be 10 to 20 times that of hydrochlorothiazide and correspondingly 100 to 200 times that of chlorothiazide. Trichlormethiazide induced sodium and chloride excretions tend to be equivalent. The possible occurrence of hypochloremic alkalosis is lessened compared to hydrochlorothiazide.Trichlormethiazide also exhibits a higher sodium-potassium ratio than either hydrochlorothiazide or chlorothiazide, thus tending to decrease the incidence of hypokalemic manifestations.
Dexamethasone is an effective corticosteroid possessing excellent anti-inflammatory activity. Dexamethasone did not cause sodium or water retention in laboratory studies.
The saluretic action of trichlormethiazide together with the anti-inflammatory activity of dexamethasone provide a complementary effect. Edema is relieved through accelerating water transport from the engorged tissue spaces without serious effects on systemic sodium levels or local electrolyte balance.
Equine: The diuretic effects of varying concentrations and combinations of trichlormethiazide and betamethasone acetate were determined in mares. Trichlormethiazide at 5 mg/45 kg (100 lbs) of body weight was shown to be an effective diuretic. When combined with 0.25 mg of betamethasone/45 kg (100 lbs), optimal diuresis was noted. Since dexamethasone and betamethasone are essentially bioequivalent, dexamethasone and trichlormethiazide will provide a similar response.
Inflammatory edema of the prepuce was induced in geldings. Injectable trichlormethiazide (5 mg) and betamethasone (0.25 mg) were administered/45 kg (100 lbs) of body weight. Comparisons were made with placebo, NAQUASONE (betamethasone and trichlormethiazide) granules and Lasix (furosemide) treated horses. Both trichlormethiazide/betamethasone products were significantly more effective than Lasix or the placebo.
Two Canadian investigators treated 66 horses affected by inflammatory edema.
The results are summarized in the following table.
INFLAMMATORY EDEMA
|
Clin. Eval. |
Not Spec. |
Bruising |
Trauma. wounds |
Allerg. React. |
Local Infect. |
Post Surg. |
Bruising & Traumatic & Post Surg. Wounds |
Trauma. Wounds & Loc. Infect. |
Total |
% |
|
Poor |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
1 |
(1.5) |
|
Fair |
0 |
2 |
4 |
1 |
1 |
3 |
1 |
0 |
12 |
(18.2) |
|
Good |
1 |
5 |
13 |
13 |
6 |
6 |
3 |
3 |
50 |
(75.8) |
|
Not Spec. |
0 |
1 |
0 |
1 |
0 |
0 |
1 |
0 |
3 |
(4.5) |
|
Total |
1 |
8 |
17 |
16 |
7 |
9 |
5 |
3 |
66 |
100 |
Bovine: A study was conducted to determine the optimum dose levels of the two ingredients in NAQUASONE injectable solution (trichlormethiazide (TCM) and dexamethasone (Dex) for production of diuresis when injected into cattle.
All possible combinations of three levels of dexamethasone (5, 10 and 20 mg/450 kg [1000 lb] of body weight) and four levels of trichlormethiazide (50, 100, 200 and 400 mg/450 kg [1000 lb] of body weight) were tested, as well as the bolus form currently marketed. The optimum combination was 20 mg of Dex and 200 mg of TCM per 450 kg (1000 lb) of body weight. The patterns of urine flow were different when comparing the injectable versus the bolus forms. In this model, the level of TCM is more closely related to the amount of urine output than is the level of dexamethasone. At least 200 mg of TCM is required to maximize output, and increasing amounts of dexamethasone slightly increase urine output.
Another study was designed to assess the amount of diuresis produced following treatment with NAQUASONE injectable solution or formulations containing the individual components thereof.
Heifers treated with NAQUASONE injectable solution consistently produced more urine than vehicle-treated controls. Animals given Dex alone excreted less urine than controls, while those injected with TCM alone produced less urine than controls in one replicate study and more urine in the other study.
The combination of ingredients in NAQUASONE injectable solution produced more diuresis than formulations of either of the individual components.
Clinical trials were conducted in Canada and France. In the principal Canadian trial, NAQUASONE injectable solution plus Bolus, NAQUASONE injectable solution alone and NAQUASONE Bolus alone were compared. Two hundred and thirty-three (233) completed case reports were received. All treatments were effective in the alleviation of signs of udder edema. A numerically but not significantly more rapid onset of activity was noted when an injection was administered. The clinician will choose the treatment regimen based upon severity of the condition. A number of cows were treated prior to parturition. One evaluator reported that five out of thirty-eight (5/38) calved prematurely. Sequellae were not severe and included one stillbirth, four (4) retained placentas and one (1) metritis. Treatment had no appreciable effect upon milk production.
SAFETY AND TOXICOLOGY: Equine: The effect of two and six times the recommended dose of NAQUASONE injectable solution (trichlormethiazide and dexamethasone) for once or twice the recommended time was determined in mares. NAQUASONE injectable solution was administered by intramuscular injection. The drug treatment produced immediate signs of steroid activity. These signs persisted for 7-14 days after treatment. There were no untoward physiological effects associated with NAQUASONE injectable solution treatment. There was moderate site of injection swelling in horses administered the highest dose. There was some swelling two weeks post the last injection. There was no swelling at twice the recommended dose. Bovine: The effect of three times the recommended dose of NAQUASONE injectable solution followed by three times the recommended dose of NAQUASONE Bolus was determined. The drug treatment produced immediate signs of steroid activity on such parameters as blood and urinary glucose, and white blood cells, and the changes persisted during the course of treatment, and from one to seven (1-7) days after the last treatment. The other parameters monitored showed no untoward physiological effects associated with treatment with NAQUASONE injectable solution.
All the physiological changes that occurred in the treated cows are to be expected with corticosteroid therapy. It appears that in lactating dairy cattle, NAQUASONE injectable solution and NAQUASONE Bolus are safe to use in tandem at three times the recommended dose.
Storage
Store between 15 and 30°C.How Supplied
NAQUASONE injectable solution, 100 ml vials.INTERVET CANADA CORP., KIRKLAND, QUÉBEC H9H 4M7, 1-888-306-0069
Rev.16NOV2009
CPN: 12080636
Intervet Canada Corp.
16750 ROUTE TRANSCANADIENNE, KIRKLAND, QC, H9H 4M7
| Order Desk: | 514-428-7013 | |
| Toll-Free: | 866-683-7838 | |
| Fax: | Toll-free 888-498-4444; local 514-428-7014 | |
| Website: | www.merck-animal-health.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
