Mirataz 2% (Canada)
This treatment applies to the following species: Company: Dechra
Company: Dechra
Mirtazapine ointment, 2% w/w
Veterinary Use Only
DIN 02504626
Description
Mirataz is a white to off-white ointment containing 2% (w/w) mirtazapine, USP (100 mg per tube) suitable for transdermal (topical) administration. Mirtazapine is an α2-adrenergic receptor antagonist, nor-adrenergic and serotonergic drug.
Mirataz 2% Indication
For body weight gain in cats with a poor appetite and weight loss.
Mirataz 2% Dosage And Administration
Administer topically by applying a 3.8 cm ribbon of ointment (approximately 2 mg/cat) on the inner pinna of the cat’s ear once daily for 14 days (see Diagrams below).
Wear disposable gloves when applying Mirataz. Dispose of used gloves after each application.
Alternate the daily application of Mirataz between the left and right inner pinna of the ears. Do not administer into the external ear canal. If desired, the inner pinna of the cat’s ear may be cleaned by wiping with a dry tissue or cloth immediately prior to the next scheduled dose. If a dose is missed, apply Mirataz the following day and resume daily dosing.
To demonstrate the method of administering the dose, the veterinarian or trained personnel at the clinic should apply the first dose in the presence of the owner.
This ruler measures 3.8 cm. Use this ruler to measure the 3.8 cm ribbon of ointment to be applied.

To apply Mirataz:
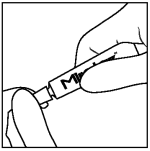
Step 1: Wear disposable gloves. Twist cap on tube counterclockwise to open.
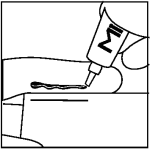
Step 2: Apply even pressure on tube and squeeze a 3.8 cm line of ointment onto your gloved finger using the measured line on the carton or on the package insert.
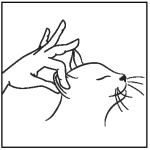
Step 3: Using your gloved finger, gently rub ribbon of ointment on inside pinna of the cat’s ear spreading it evenly over the surface. Dispose of used gloves after each application. If contact with your skin occurs wash thoroughly with soap and water.
Contraindications
Mirataz is contraindicated in cats with a known hypersensitivity to mirtazapine or to any of the excipients.
Mirataz should not be given in combination, or within 14 days before or after treatment with a monoamine oxidase inhibitor (MAOI) [e.g. selegiline hydrochloride (L-deprenyl), amitraz], as there may be an increased risk of serotonin syndrome.
Mirataz 2% Cautions
Do not administer orally or to the eye.
A poor appetite is a common clinical sign associated with many systemic diseases. Appropriate diagnosis and treatment of the underlying cause should be addressed.
Use with caution in cats with hepatic disease. Mirtazapine may cause elevated serum liver enzymes (See Animal Safety).
Use with caution in cats with kidney disease. Kidney disease may cause reduced clearance of mirtazapine which may result in higher drug exposure.
It is important to carefully monitor body weight, body condition score and food intake during treatment. In cats that continue to lose weight, alternative treatments should be considered.
Upon discontinuation of Mirataz, it is important to monitor the cat’s food intake. Food intake may lessen after discontinuation of mirtazapine transdermal ointment. If food intake diminishes dramatically (>75%) for several days, or if the cat stops eating for more than 48 hours, reevaluate the cat.
Mirataz has not been evaluated in cats < 2 kg or less than 7.5 months of age. The safe use of Mirataz has not been evaluated in cats that are intended for breeding, pregnant, or lactating cats.
Warnings
Keep out of reach of children. When handling the product, wear disposable latex or nitrile gloves to prevent accidental topical exposure. Dispose of used gloves properly and wash hands with soap and water after application. Care should be taken that people or other animals in the household do not come in contact with the treated cat for at least 2 hours after application because mirtazapine can be absorbed transdermally and orally.
People with known hypersensitivity to mirtazapine should not handle this product. Due to limited data on the reproductive toxicity of mirtazapine, pregnant women or women trying to conceive should avoid handling this product.
In case of accidental skin exposure, wash thoroughly with soap and water. In case of accidental eye exposure, flush eyes thoroughly with water. In case of accidental ingestion or if skin or eye irritation occurs, seek medical attention immediately.
Adverse Reactions
Although all adverse reactions are not reported, the following information is based on voluntary post-approval drug experience reporting. It is generally recognized that this results in significant under-reporting. The adverse events listed here reflect reporting and not necessarily causality. Adverse events are listed by body system, in decreasing order of frequency:
Application site disorders: application site erythema, application site lesions, application site reactions NOS, application site inflammation.
Systemic disorders: lack of efficacy, anorexia, lethargy.
Behavioural disorders: vocalization, hyperactivity.
Ear and labyrinth disorders: external ear disorder NOS.
Digestive tract disorders: emesis, hypersalivation.
Neurological disorders: ataxia.
In a randomized, double-masked, vehicle-controlled field study to assess the efficacy and safety of mirtazapine for the management of weight loss in cats, 115 cats treated with Mirataz and 115 cats treated with vehicle control were evaluated for safety. The vehicle control was an ointment containing the same inert ingredients as Mirataz without mirtazapine. The most common adverse reactions included application site reactions, behavioral abnormalities (vocalization and hyperactivity), and vomiting. The adverse reactions observed in the study and number of cats experiencing each adverse reaction is summarized in Table 1 below.
Table 1. Adverse Reactions Reported During the Field Study
|
Adverse Reaction |
Mirataz |
Vehicle Control |
|
Application site (Ear pinna) |
||
|
Erythema |
12 (10.4%) |
20 (17.4%) |
|
Crust/Scab |
3 (2.6%) |
6 (5.2%) |
|
Residue |
3 (2.6%) |
8 (7.0%) |
|
Scaling/Dryness |
3 (2.6%) |
3 (2.6%) |
|
Dermatitis or irritation |
1 (0.9%) |
9 (7.8%) |
|
Alopecia |
1 (0.9%) |
2 (1.7%) |
|
Pruritus |
1 (0.9%) |
4 (3.5%) |
|
Behavioral |
||
|
Vocalization |
13 (11.3%) |
2 (1.7%) |
|
Hyperactivity |
8 (7.0%) |
1 (0.9%) |
|
Disoriented state or ataxia |
4 (3.5%) |
2 (1.7%) |
|
Lethargy/weakness |
4 (3.5%) |
9 (7.8%) |
|
Attention seeking |
3 (2.6%) |
0 |
|
Aggression |
2 (1.7%) |
0 |
|
Physical Examination or Observational |
||
|
Vomiting |
13 (11.3%) |
15 (13.0%) |
|
Dehydration |
6 (5.2%) |
5 (4.3%) |
|
Diarrhea or soft stool |
6 (5.2%) |
7 (6.1%) |
|
Heart murmur |
5 (4.3%) |
7 (6.1%) |
|
Inappetence |
5 (4.3%) |
5 (4.3%) |
|
Renal insufficiency* |
4 (3.5%) |
0 |
|
Ear infection |
3 (2.6%) |
0 |
|
Urinary tract infection |
3 (2.6%) |
0 |
|
Clinical Pathology |
||
|
Hematuria |
7 (6.1%) |
1 (0.9%) |
|
Elevated BUN (without creatinine**) |
6 (5.2%) |
0 |
|
Elevated creatinine and BUN |
5 (4.3%) |
1 (0.9%) |
|
Hyperphosphatemia |
5 (4.3%) |
0 |
|
Hypokalemia |
5 (4.3%) |
2 (1.7%) |
|
Pyuria |
5 (4.3%) |
0 |
|
Anemia |
3 (2.6%) |
8 (7.0%) |
|
Low urine specific gravity |
3 (2.6%) |
1 (0.9%) |
|
Monocytosis |
3 (2.6%) |
2 (1.7%) |
|
Neutrophilia |
3 (2.6%) |
2 (1.7%) |
* One cat with renal insufficiency was reported with a serious adverse reaction of acute renal failure, hematuria, and pyuria at the Week 2 visit. The cat was enrolled with a history of chronic kidney disease. Euthanasia was elected and necropsy revealed hypertrophic cardiomyopathy, bilateral parathyroid hyperplasia, and mild to moderate renal disease.
** At Week 2, blood urea nitrogen (BUN) values were significantly higher in the Mirataz group compared to the vehicle control group (p<0.10). The BUN in the Mirataz group was 15.6 mmol/L (reference range 5.7-13.2 mmol/L) compared to 12.9 mmol/L in the vehicle control group.
Clinical Pharmacology
Mechanism of action: The exact mechanism by which mirtazapine induces weight gain has not been clearly elucidated but appears to be multifactorial. Mirtazapine is an α2-adrenergic receptor antagonist nor-adrenergic and serotonergic antidepressant drug. Mirtazapine is known to be a potent antagonist of 5-HT2 and 5-HT3 serotonin receptors in the central nervous system (CNS), and a potent inhibitor of histamine H1 receptors. Mirtazapine is thought to antagonize the 5-HT2 and H1 histamine receptors, which both play a role in appetite regulation. The weight gain seen with mirtazapine may also be secondary to changes in leptin and the tumor necrosis factor (TNF) cytokine system.
Pharmacokinetics: Mirtazapine is absorbed transdermally after application to the inner pinna of the ear. There are large inter-individual pharmacokinetic variations, larger than after oral administration. The absorption is variable between individuals with sustained plasma concentrations of mirtazapine and a prolonged half-life. Plasma mirtazapine concentrations peak between 1 and 4 hours after dosing. In a crossover study in eight cats to determine the relative bioavailability of oral and transdermal 2% mirtazapine, the mean half-life (26.8 hours) with topical administration was over 2X longer than the mean half-life (10.1 hours) with oral administration.
Safety And Efficacy Information
Safety: In a 6-week laboratory safety study, 48 healthy cats aged 7-10 months were dosed topically with mirtazapine once daily at target doses of 0 mg/kg (vehicle control), 1 mg/kg, 3 mg/kg, and 5 mg/kg body weight. Four cats/sex/group in the 1 and 3 mg/kg groups were dosed topically to the inner pinna of the ear, alternating between right and left ears. Eight cats/sex/group in the 0 and 5 mg/kg groups were dosed topically to the inner pinna of the ear, splitting the dose between both ears. Four cats/sex/group in the 0 and 5 mg/kg groups were maintained and monitored during a 4-week recovery period.
6 Week Dosing Period:
Application of mirtazapine and vehicle control was associated with ear flicking, head shaking, pulling away/flinching and infrequently with struggling/fractious behavior, and hypersalivation.
Inner and outer pinna erythema, flaking, alopecia, and thickening were observed in all cats in all groups. Erythema, crusting, alopecia, and scabbing of the skin, mostly around the head and neck, was frequently observed in all groups and occasionally affected the tail, tarsi or carpi, likely due to spread of the ointment to these areas by self-grooming.
Mirtazapine administration resulted in increased vocalization, decreased activity, and inconsistent changes in attention-seeking behaviors in all mirtazapine dose groups. Vomiting was observed in 25% of the control, 1 and 5 mg/kg cats and 50% of the cats in the 3 mg/kg group. Polyuria was observed in all groups.
Polydipsia was observed in one cat in the 5 mg/kg group. Three cats (one from each mirtazapine dose group) were isosthenuric. Eight cats developed cystitis with or without urethral obstruction throughout the study in all groups. One cat each from the vehicle control and 3 mg/kg group were euthanized early on Day 35 due to urethral obstruction.
Two cats from the 1 mg/kg group had either ventricular premature contractions (VPC) or tall R waves, and one cat from the 5 mg/kg group had both VPC and a right axis deviation.
Mild elevations in ALT values were noted sporadically in vehicle control, 3, and 5 mg/kg groups. On Day 15, one cat in the 3 mg/kg group had a marked ALT elevation of 3397 U/L, with concurrent elevations in AST and GGT. By Day 42, the ALT declined to 109 U/L and the AST and GGT returned to within normal limits. Gross pathology findings, confirmed with histopathology, were hyperplastic dermatitis (alopecia, hyperkeratosis, thickening, and ceruminous gland secretion) of the pinnae in all cats and findings consistent with cystitis (mucosal urinary bladder hemorrhage, mottled-dark red appearance, and irregular contour) in four male cats (two vehicle control, one 3 mg/kg and one 5 mg/kg). Additional histopathologic findings included pyelonephritis (two vehicle control and one 5 mg/kg), nephrocalcinosis (three vehicle control, one 1 mg/kg, three 3 mg/kg, and one 5 mg/kg), and necrosis of the kidneys (one vehicle control and one 3 mg/kg).
4 Week Recovery Period:
Following a 4-week recovery period, ALT elevations resolved. Polyuria was reduced and only occurred in two cats in the 5 mg/kg group. Pinnal lesions (erythema and flaking) completely resolved in the vehicle control and improved in the 5 mg/kg groups. Ear thickening improved in both groups.
Efficacy: The efficacy of Mirataz (mirtazapine transdermal ointment) was demonstrated in a randomized, double-masked, vehicle-controlled, multi-site field study involving client-owned cats of various breeds. Enrolled cats had to be 1 year of age or older and had existing documented medical history of 5% weight loss deemed clinically significant. The most common pre-existing conditions included renal insufficiency, vomiting, and hyperthyroidism. Some cats had more than one pre-existing condition. Cats were randomized to treatment groups in a 1:1 ratio of Mirataz to vehicle control. A total of 230 cats were enrolled and received either Mirataz (115 cats) or a vehicle control (115 cats) containing the same inert ingredients without mirtazapine. The median age of the cats was 14.2 years and the median weight of the cats was 4.1 kg. The dosage was a 3.8 cm ribbon (approximately 2 mg/cat) mirtazapine or vehicle ointment administered topically to the inner pinna of the cat’s ear.
A total of 177 cats were determined to be eligible for the effectiveness analysis; 83 cats were in the Mirataz group and 94 cats were in the vehicle control group. The primary effectiveness endpoint was the mean percent change in body weight from Day 1 to the Week 2 visit.
At Week 2, the mean percent increase in body weight from Day 1 was 3.94% in the mirtazapine group and 0.41% in the vehicle control group. The difference between the two groups was significant (p<0.0001) based on a two-sample t-test assuming equal variances. A 95% confidence interval on the mean percent change in body weight for the Mirataz group is (2.77, 5.11), demonstrating that the mean percent change is statistically different from and greater than 0.
Storage
Store below 25°C. Discard within 30 days of first use.
PRESENTATION
Mirataz is supplied in a 5 g multi-use tube.
Dechra Ltd., Snaygill Industrial Estate, Keighley Road, North Yorkshire, United Kingdom, BD23 2RW
Imported and Distributed by:
Dechra Veterinary Products Inc., 1 Holiday Ave, East Tower, Suite 345, Pointe-Claire, Quebec, H9R 5N3, Canada
141168
CPN: 1786065.0
1 HOLIDAY AVE., EAST TOWER SUITE 345, POINT-CLAIRE, QC, H9R 5N3
| Toll-Free: | 855-332-9334 | |
| Technical Services: | 855-332-9334 Option 1 | |
| Technical Services Email: | technical.ca@dechra.com | |
| Website: | www.dechra.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
