Metacam Oral Suspension for Dogs (Canada)
This treatment applies to the following species: Company: Boehringer Ingelheim Animal Health
Company: Boehringer Ingelheim Animal Health
Veterinary Use Only
DIN 02237715
Composition
Each mL contains 1.5 mg meloxicam in a yellowish suspension with an odour of honey.Presentation: Plastic squeeze dropper bottle containing either 10, 32, 100 or 180 mL.
A Metacam® measuring syringe is also provided in the pack.
Metacam Oral Suspension for Dogs Indications
Metacam® is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam group for use in dogs. It acts by inhibition of prostaglandin synthesis and is indicated for the alleviation of inflammation and pain in both acute and chronic musculo-skeletal disorders.
Dosage: Metacam® Oral Suspension should be administered mixed with foods. On the first day of treatment, a single dose of 0.2 mg meloxicam/kg bodyweight should be given. Treatment is to be continued once daily by oral administration (at 24 hour intervals) at a maintenance dose of 0.1 mg meloxicam/kg body weight.
Owners should be advised when their dog has received a meloxicam injection, and be informed of the potential for adverse reactions and clinical signs associated with NSAID intolerance. Always provide client information sheet with prescription. Dogs undergoing prolonged treatment with Metacam® should be monitored periodically.
Instructions for use: Particular care should be given with regard to the accuracy of dosing. The suspension can be given using either the drop dispenser (for very small breeds) - which provides 0.05 mg meloxicam per drop - or the Metacam® measuring syringe provided in the package (see below). The syringe fits onto the bottle and has a kg-body weight scale designed for the maintenance dose (i.e. 0.1 mg meloxicam/kg body weight). Thus twice the volume should be administered on the first day as the initial dose. Shake well before use. Please carefully follow the instructions of the veterinarian.
Dosing procedure using the measuring syringe:
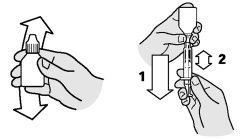
Shake well!
1. Firmly attach the measuring syringe to the bottle tip
2. Pull the plunger back to the approximate dosage volume required
3. Adjust dosage volume to the corresponding body weight
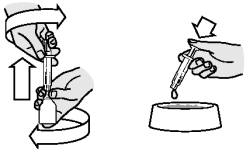
Twist the bottle and the syringe in opposite directions and pull apart
Empty the contents of the syringe over the feed
Improvement is normally seen within 3-4 days. Treatment should be discontinued after 10 days at the latest if no improvement is apparent.
Contraindications
Metacam® should not be administered if gastric or intestinal ulceration or bleeding is suspected; if there is evidence of cardiac, hepatic or renal disease; or if there is evidence of a haemorrhagic disorder or individual hypersensitivity to the product. Do not administer concurrently, other steroidal or nonsteroidal anti-inflammatory drugs (NSAIDs), aminoglycoside antibiotics or anticoagulant agents.
Pre-treatment with other steroidal or nonsteroidal anti-inflammatory drugs (NSAIDs) may result in additional or increased effects and accordingly a treatment-free period with such drugs should be observed for at least 24 hours before commencement of treatment depending on the pharmacokinetic properties of the products used previously.
Do not use if there is evidence of dehydration, hypovolemia or hypotension, because of the increased risk of renal injury caused by the destruction of protective prostaglandins secreted by the kidneys in this risk situation.
Cautions: Not approved for use in cats.
Metacam® should not be administered to breeding, pregnant or lactating dogs. Do not exceed the stated dose. In case of overdosing, symptomatic treatment should be initiated.
Animals being treated with meloxicam should be monitored for the occurrence of side effects as susceptibility varies with the individual.
If vomiting, inappetence, lethargy, diarrhea, increased drinking, increased or inappropriate urination or other suspected adverse reactions occur, IMMEDIATELY discontinue treatment and seek the advice of a veterinarian (see Adverse Reactions).
As for all NSAIDs, use in any animal less than 6 weeks of age or in debilitated aged animals may involve additional risk. If use in such animals cannot be avoided; a reduced dosage and careful clinical management may be required.
Warnings
- Keep out of reach of children.
- People with known hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) should not handle this product.
- Pregnant women should not handle this product unless adequate exposure protection can be assured.
- Caution should be taken to avoid accidental ingestion and contact with eyes.
- This product can cause eye irritation. In case of contact with the eyes, immediately rinse thoroughly with water.
Adverse Reactions
Postmarketing reports of suspected adverse drug reactions (SADRs) following field use of Metacam® (meloxicam) have been monitored worldwide since 1995.Typical adverse reactions of NSAIDs, such as loss of appetite, vomiting, diarrhea, apathy or polyuria and polydipsia associated with renal failure have occasionally been reported. These adverse effects are in most cases transient and disappear following termination of the treatment but in very rare cases may be serious or fatal.
Although not all adverse reactions are reported, the following adverse reaction information is based on voluntary post-approval drug experience reporting by veterinarians and pet owners/caregivers. It should be noted that suspected adverse drug reactions listed here reflect reporting and not causality. The categories of adverse reactions are listed in decreasing order of frequency by body system. In rare cases, death has been associated with some of these adverse reactions.
Digestive tract disorders: Vomiting, diarrhea, melena, hematemesis, ulceration.
Systemic disorders: Inappetence, lethargy, jaundice.
Neurological disorders: Ataxia, seizures, trembling.
Behavioural disorders: Behavioural disorder (not otherwise specified), hyperactivity.
Renal and urinary disorders: Acute renal failure.
Investigations: Elevated creatinine and BUN, elevated liver enzymes.
Skin and appendages disorders: Pruritus, eczema, alopecia local, moist dermatitis (hot spots), allergic dermatitis.
Immune system disorders: Urticaria, immune mediated hemolytic anemia, immune mediated thrombocytopenia.
Information for Pet Owners: Metacam® (meloxicam) is a nonsteroidal anti-inflammatory drug (NSAID) and as with other drugs in this group, adverse reactions may occur in treated dogs. The most common adverse effects reported involve the gastrointestinal tract and the kidneys and usually occur within the first week of treatment. Typical symptoms include loss of appetite, vomiting, diarrhea, dark stools, depression, increased drinking and increased or inappropriate urination. It is important in these situations to discontinue treatment IMMEDIATELY and contact your veterinarian. In most cases, the adverse reactions are transient and disappear after termination of treatment but in rare instances may be serious especially if treatment is not discontinued.
Target Animal Safety: The safety profile of meloxicam has been evaluated in well-controlled target animal safety studies in the dog. Dogs treated with placebo, 1X, 3X and 5X label dosages were closely monitored over a 180 day (26 weeks) period. The study determined that there were no drug related adverse effects on clinical observations, normal body weight gain, food consumption, physical and ophthalmic examinations, clotting times, mucosal bleeding times or on a panel of clinical pathology parameters monitored throughout the study.
Shake well before using.
Store at or below 25°C.
Boehringer Ingelheim Animal Health Canada Inc., Burlington ON L7L 5H4
Metacam® is a registered trademark of Boehringer Ingelheim Vetmedica GmbH, used under license.
Revised: 11-2022
159488-004
CPN: 1182167.1
5180 SOUTH SERVICE ROAD, BURLINGTON, ON, L7L 5H4
| Customer Care No.: | 1-800-567-1885 | |
| Technical Services No.: | 1-877-565-5501 | |
| Website: | www.boehringer-ingelheim.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
