HemaBlock
This treatment applies to the following species:STOP the bleed
Description
HemaBlock Hemostatic Powder is a medical device intended for the application to surgical wound sites as an absorbable hemostatic agent. This technology incorporates hydrophilic, flowable, microporous particles synthesized from purified plant starch through a proprietary process: HemaBlock is a 100% plant based polysaccharide derived from a combination of potato starch particles and oxidized cellulose micro-fibers derived from cotton. HemaBlock contains no animal components. HemaBlock is a fine dry sterilized powder that is biocompatible, non-pyrogenic and is typically absorbed within 3-5 days. Applications include general and oral surgical hemorrhage control, mass removals, donor sites, biopsies, enucleations, dental extractions and flaps, suture lines and topical trauma wounds such as ear, tail and pad lacerations and nail trims.
Action: The active component in HemaBlock am Microporous Polysaccharide Beads (MPB) which are hydrophilic molecular sieves that enhance natural hemostasis by concentrating blood solids such as platelets, red blood cells and blood proteins on the particle surface to form a gelled matrix. The concentrated gel matrix provides a barrier to further blood loss and is formed regardless of the patient’s coagulation status. The concentration of dotting factors and platelets in the gel serves to enhance normal clotting reactions and creates stable hemostatic plugs. The absorption process begins immediately and is dependent on several factors, including the amount applied and the site of use.
HemaBlock Indications
HemaBlock Hemostatic Powder is a hemostatic device that controls capillary, venous, and arteriolar bleeding on companion animals, horses and exotic species.
Contraindications
Do not inject or place HemaBlock inside blood vessels as potential for embolization and death may exist.
Warnings
HemaBlock is not intended as a substitute for meticulous surgical technique and proper application of ligatures or other conventional procedures for hemostasis. Once hemostasis is achieved, excess powder should be flushed from the wound site by irrigation particularly when used around the spinal cord or the optic nerve. HemaBlock swells to its maximum volume immediately upon contact with blood or other fluids. The possibility of product interfering with normal function and/or causing compression necrosis of surrounding tissue due to swelling is reduced by removal of excess dry material. Safety and effectiveness in ophthalmic procedures has not been established. Avoid using on the surface of the eye.Precautions
HemaBlock is intended to be used in a dry state. Contact with saline or other solutions prior to achieving hemostasis will result in the loss of hemostatic potential.HemaBlock is supplied as a sterile product and cannot be re-sterilized. Unused, open containers of HemaBlock should not be used in a sterile procedure.
Directions For Use
Inspect the integrity of the HemaBlock packaging prior to use. If damaged, do not use. For the foil sachet in the re-sealable tube, remove the tamper evident cap ring and open tube to drop sterile sachet contents on the OR tray to maintain the sterile field. For the Prefilled Syringe applicator, peel open chevron seal on pouch end and drop applicator on OR tray to maintain sterile field. For maximum benefit, the following technique is recommended:
Remember the acronym of R. A. P. I. D. Hemostasis
Instructions for Use
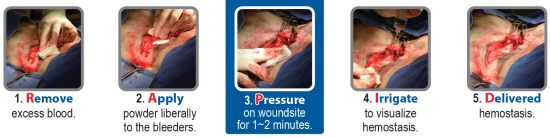
1. Remove excess blood by blotting, wiping or suction. It is important to apply HemaBlock directly to the site of the active bleeding.
2. Apply a liberal amount of the powder as close to the source of the bleeding as possible and completely cover the wound. Deep wounds like biopsy sites may require equally deep application of the HemaBlock powder. To minimize possible occlusion of the applicator tip, pressure should be applied as the applicator exits the wound.
3. Steady Pressure needs to be applied over the powder covered tissue for 1 to 2 minutes - use of a non-adhering substrate to apply pressure may prevent adhesion of the formed clot to the surgical glove or other instrumentation. Amount and duration of pressure is wound dependent. For oozing, pressure may not be necessary. For more profuse bleeding wounds, steady pressure should be maintained longer. If bleeding continues, remove excess HemaBlock powder and reapply.
4. Irrigate wound area with saline and carefully clear away any material (excess powder, glove, sponge, pad, etc.) to visualize hemostasis. Excess HemaBlock powder not entrained in the clot matrix will swell upon contact. Once hemostasis is achieved the excess material should be removed by additional irrigation and aspiration.
5. Delivered hemostasis is evident after irrigation and the wound site is then ready for traditional closure methods.
Administration: Aseptic technique should always be used. A liberal amount of the HemaBlock powder should be applied to the bleeding site followed by steady pressure until hemostasis is achieved. After hemostasis, any excess powder should be deactivated by irrigation and removed from the site. Opened containers of HemaBlock powder should not be reused for other sterile procedures but can be applied for non-sterile applications.
How Supplied
HemaBlock Hemostatic powder is supplied in a 2 gram foil sachet which is packaged in a re-sealable poly tube and is also available in a 0.5 gram prefilled 1 cc modified syringe applicator. The 2 gram sachet has been tested on various wound sites to provide wound coverage of approximately 30 cm2 area or over 50 cm of suture line coverage. The syringe applicator is appropriate for one large biopsy site or tooth extraction site or multiple smaller wound sites and will cover about 6cm2 of wound area.Storage and Handling: HemaBlock powder should be stored to avoid temperature extremes (less than -40C/F and greater than 140F/60C). Once the package is open, contents are subject to moisture uptake or contamination and should be used immediately. Storage of an opened foil sachet back in the original capped outer tube is appropriate for secondary non-sterile applications for the excess unused powder.
Disclaimer: Hemablock, LLC shall not be liable for any direct, incidental, or consequential damages other than as expressed by specific law. No individual has the authority to bind Hemablock, LLC to any representation or claim except as specifically set forth herein. Descriptions or specifications are presented to generally describe HemaBlock powder and related procedures, and do not constitute any expressed claims or practices.
Manufactured in the USA by Hemablock, LLC, for Vet Brands International Miramar, FL
E-mail address: sales@vetbrands.com
Phone number: 954.392.6072
www.hemablock.com
02032015
CPN: 6163008.0
10467 NORTH COMMERCE PARKWAY, MIRAMAR, FL, 33025
| Telephone: | 954-392-8072 | |
| Toll-Free: | 800-766-7543 | |
| Fax: | 954-392-8076 | |
| Website: | www.vetbrands.com | |
| Email: | info@vetbrands.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
