Genesis Topical Spray
This treatment applies to the following species: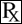 Company: Virbac
Company: Virbac
Solution of 0.015% triamcinolone acetonide
FOR TOPICAL USE IN DOGS ONLY
FOR THE CONTROL OF PRURITUS ASSOCIATED WITH ALLERGIC DERMATITIS IN DOGS
Approved by FDA under NADA # 141-210
Genesis Topical Spray Caution
Federal law (USA) restricts this drug to use by or on the order of a licensed veterinarian.
Description
GENESIS® Topical Spray contains 0.015% triamcinolone acetonide for dermatologic use. Each mL of GENESIS Topical Spray contains 0.15 mg triamcinolone acetonide in an aqueous solution containing propylene glycol, specially denatured alcohol, and DMDM hydantoin.
Pharmacology
Triamcinolone acetonide is a highly potent synthetic glucocorticoid, which is primarily effective because of its anti-inflammatory activity. Topical corticosteroids can be absorbed from normal intact skin. Studies have demonstrated that topical preparations of triamcinolone have decreased plasma cortisol levels and suppressed the response to ACTH.
Genesis Topical Spray Indications
GENESIS Topical Spray is indicated for the control of pruritus associated with allergic dermatitis in dogs.
Genesis Topical Spray Dosage And Administration
Apply sufficient pump sprays to uniformly and thoroughly wet the affected areas while avoiding run-off of excess product. Avoid getting the spray in dog’s eyes. GENESIS Topical Sprays should be administered twice daily for seven days, once daily for the next seven days, then every other day for an additional 14 days (28 days total).
To avoid overdosing the product, use the following table to determine the maximum number of pump sprays per treatment application. For mild pruritus or for small treatment surface areas, the number of pumps used should be less than this maximum amount.
Table 1. Maximum allowable dosage
|
Dog weight |
Maximum number of pumps per single application* |
Total maximum volume (mL) per 28 day treatment regimen |
|
|
lb |
kg |
||
|
11 |
5 |
4 |
101 |
|
22 |
10 |
7 |
176 |
|
33 |
15 |
11 |
277 |
|
44 |
20 |
15 |
378 |
|
55 |
25 |
19 |
478 (one 16-oz bottle) |
|
66 |
30 |
22 |
554 |
|
77 |
35 |
26 |
655 |
|
88 |
40 |
30 |
756 |
|
99 |
45 |
33 |
832 |
|
110 |
50 |
37 |
932 (two 16-oz bottles) |
*Using the recommended dosing regimen, there are two applications per day for the first week, one application per day for the second week and one application every other day for the last two weeks of treatment.
Warnings
User Safety: Wear gloves when applying the product. Spray in a well ventilated area. If the spray causes irritation to mucous membranes, discontinue use.
Keep this and all drugs out of reach of children.
Animal Safety: Clinical and experimental data have demonstrated that corticosteroids administered orally or by injection to animals may induce the first stage of parturition if used during the last trimester of pregnancy and may precipitate premature parturition followed by dystocia, fetal death, retained placenta, and metritis. Additionally, corticosteroids administered to dogs, rabbits, and rodents during pregnancy have resulted in cleft palates in offspring. Corticosteroids administered to dogs during pregnancy have also resulted in other congenital anomalies including deformed forelegs, phocomelia, and anasarca.
Precautions
The safety of this product for dogs less than eight pounds or for dogs less than one year of age has not been evaluated. The safety of this product in breeding, pregnant or lactating dogs has not been evaluated (see WARNINGS). The safety of long term or repeated use of this product (greater than 28 days) has not been evaluated. Prolonged use or overdosage of any corticosteroid may produce adverse effects. Because absorption of triamcinolone acetonide through topical application on the skin and by licking may occur, dogs receiving triamcinolone acetonide therapy should be observed closely for evidence of hypothalamic-pituitary-adrenal (HPA) axis suppression. When the product was applied at approximately 6 times the maximum allowable dose (100 mL) once daily to normal skin of two dogs for five days, plasma cortisol levels were decreased after the first treatment and response to ACTH was reduced.
If adverse clinical signs are observed, treatment should be discontinued. Once the signs have disappeared, treatment can be resumed at a lower dose or frequency of application. If hypersensitivity to the product occurs, treatment should be discontinued and appropriate therapy instituted. In the presence of dermatological infections, use of an appropriate antifungal or antibacterial agent should be instituted. If a favorable response does not occur promptly, the corticosteroid should be discontinued until the infection has been adequately controlled.
Adverse Reactions
In a field study with GENESIS Topical Spray, polyuria was reported in 3 of 57 dogs (5.3%) and polyphagia in 1 of 57 dogs (1.8%). Mild (within reference range) decreases in total leukocyte, lymphocyte and eosinophil counts were also reported. The following local reactions were reported in ≤ 3.6% of 110 dogs treated with GENESIS Topical Spray or the product vehicle: aversion/discomfort, sneezing and watery eyes.
Effectiveness
In a 28-day field study to demonstrate the effectiveness of GENESIS Topical Spray in controlling pruritus associated with allergic dermatitis in dogs under field conditions, 105 dogs with atopy, unspecified allergic dermatitis, flea allergy, and food allergy were treated with GENESIS Topical Spray at the recommended use level or placebo. Results are shown in Table 2.
Table 2. Percent of cases considered treatment successes
|
Treatment |
Percent success1 |
|
GENESIS Topical Spray |
35/54 = 64.8%* |
|
Placebo |
12/51 = 23.5% |
1Success = reduction in the level of severity by two or more grades in the investigator’s overall evaluation from the pre-treatment to the post-treatment evaluation period.
*Significantly different from placebo at p < 0.05
Storage Conditions
Store at room temperature, 15° - 30° C (59° - 86° F).
How Supplied
GENESIS Topical Spray is supplied in 8 ounce (237 mL) and 16 ounce (478 mL) bottles with spray applicators.
For technical information or to report adverse reactions, please call 1-800-338-3659.
Distributed by:
Virbac AH, Inc., Fort Worth, TX 76137
© 2021 Virbac Corporation. All rights reserved.
GENESIS is a registered trademark of Virbac AH, Inc.
|
Net Content: |
|
|
8 fl oz (237 mL) |
750032-05 301751-05 Rev. 10/21 |
|
16 fl oz (478 mL) |
750033-05 301752-05 Rev. 10/21 |
CPN: 1023087.4
Virbac Corporation
P.O. BOX 162059, FORT WORTH, TX, 76161
| Telephone: | 817-831-5030 | |
| Order Desk: | 800-338-3659 | |
| Fax: | 817-831-8327 | |
| Website: | https://us.virbac.com/ | |
| https://vet-us.virbac.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
