EIA AGID Test Kit
This treatment applies to the following species:EQUINE INFECTIOUS ANEMIA VIRUS ANTIBODY TEST
Principles of the Test:
The Equine Infectious Anemia Virus (EIAV) test is an Agar Gel Immuno-Diffusion (AGID) assay designed to detect the presence of antibodies against EIAV in equid serum. The test uses a recombinant protein consisting of the EIAV p26 core protein to detect the specific antibodies to EIAV. Samples are added to 1% Noble Agar in a petri dish cut with a seven well template where EIAV Recombinant p26 Antigen is in the center well and EIAV Positive Control Reference Serum is added to every second well for a total of three EIAV Positive Control Reference Serum wells for each seven well template. The remaining three wells contain test samples. Samples positive for EIAV specific antibodies react with the recombinant antigen to form an antibody antigen complex which is visualized as a precipitin line between the test well and the antigen well. Weaker samples may not form a complete precipitin line but instead change the migration pattern inward towards the sample well. Samples negative for EIAV specific antibodies will not form a precipitin line with the p26 antigen, nor will they cause a deviation of the EIAV Positive Reference Control Serum lines.
Materials Provided: All materials are provided ready-to use.
1. EIAV Recombinant p26 Antigen in diluent with stabilizing proteins and Sodium Azide as a preservative - 4 ml
2. EIAV Positive Control Reference Serum with stabilizing proteins and Sodium Azide as a preservative - 11 ml
These reagents are sufficient to perform up to 200 tests.
Materials Required But Not Provided:
1. Balance
2. Micropipettor with disposable tips
3. Hot Plate or Microwave
4. 1L flask
5. Distilled or de-ionized water
6. Boric Acid
7. Sodium Hydroxide
8. Noble Agar
9. 100 mm diameter plastic petri dish with lids
10. EIA immunodiffusion cutter
11. Vacuum pump
12. High Intensity narrow-focus light source
Precautions
1. Do not mix reagents of one kit with those from another serial or lot number.
2. Do not use after the expiration date shown on the package label.
3. For animal use only and for sale only to USDA/APHIS approved laboratories.
4. Treat all reagents and samples as potentially hazardous.
5. Warm reagents to room temperature (15-25 °C) prior to each use.
Storage and Stability:
All materials provided in the test kit should be stored at 2-8 °C.
Specimen Collection and Preparation:
Equine serum is recommended for use with this test kit. Whole blood samples should have the serum fraction separated by centrifugation and decantation.
Serum separators may also be used. Anticoagulants are not recommended.
Specimens may be stored, on or off the clot, at refrigerator temperature (2-8 °C) for up to 21 days. Samples stored on the clot should have the serum fraction separated by centrifugation and decantation prior to use. If longer storage is needed, serum should first be separated from the clot and then specimens frozen at -20 °C. Repeated freezing and thawing should be avoided. Frozen samples should thaw at room temperature (15-25 °C) and should be mixed by gentle inversion before testing begins.
Preparation of Agar Gel:
1. 9 grams of Boric Acid (H3B03 FW=61.83 g/M) is dissolved in 1 liter distilled water.
2. 2 grams of Sodium Hydroxide (NaOH FW= 40 g/M) is added to the borate buffer and allowed to mix until in solution. Alternatively a 50 x stock of sodium hydroxide may be used (2.5 M NaOH in distilled water). The final concentration of the solution is 0.146 M Boric Acid, 0.05 M NaOH. The pH of the solution is measured. Acceptable range is pH 8.6 ± 0.2.
3. 10 grams of Noble Agar (1% w/v) is added to the solution and is mixed for a few minutes until it is in a homogenous suspension.
4. The suspension is then heated by microwave or hot plate until the agar is in solution.
5. The solution is allowed to cool for a few minutes and 15 mL is added to 100 mm diameter plastic petri dishes.
6. The agar is allowed to cool until it becomes firm, the lids are placed on the plates and they are then stored inverted at 2-8 °C, for up to 7 days, until use.
Cutting the Agar Gel:
A 7 well, EIA immunodiffusion template, is used to form well patterns. There is one center well with six surrounding wells, 2.4 mm apart and 5.3 mm wide. Once cut, the plugs are removed using a cannula, or equivalent, under vacuum to create the well pattern. If moisture is present in the wells, it should be removed or evaporated prior to addition of reagents and samples. Gels should be cut on the same day they are to be used.
Preliminary Steps to Running the Assay:
1. Remove the test kit from the refrigerator about one hour before use to allow reagents to come to room temperature (15-25 °C).
2. Use a single well for each serum specimen.
3. Identify and record a well location for each sample.
Testing Procedure:
1. Add 50 µL of EIAV Recombinant p26 Antigen (Ag) to center well of the seven well pattern so that the solution is level with the top of the well.
2. Add 50 µL of EIAV Positive Control Reference Serum (RS) to every other well on the outside of the antigen well so that the solution is level with the top of the well.
3. Add 50 µL of sample (S1-S3) to the remaining three wells in the pattern so that the solution is level with the top of the well.
a. A total of 3 samples may be tested per well pattern. If less than 3 samples are to be tested, fill remaining sample wells with a blank, such as Phosphate Buffered Saline (PBS).
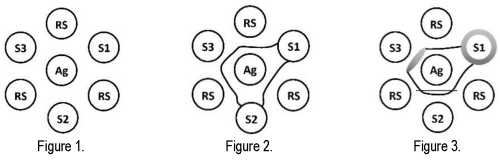
b. NOTE: See Figure 1 for loading of the well pattern.
4. Incubate at room temperature (15-25 °C) in a closed humid chamber.
5. Read plate and record results at 24 hours. If the reaction is complete after 24 hours, results may be reported. Weak positive samples may require up to 48 hours before the reaction is complete.
Quality Control:
For a test to be considered valid, the reference lines must be easily visualized using a high intensity narrow-focus light source. The termination of the lines, at the sample well for negative and weak positive or as a continuous line between the Reference Serum and Sample well, must be clearly identifiable.
NOTE: termination lines may not be fully visual at 24 hours and therefore later interpretation may also be required.
Results:
A high intensity narrow-focus light source should be used for interpretation. Read the gels against a black background or in dark ambient light. Interpret reference lines according to the following:
1. Positive - Positive Control Reference Serum (RS) lines join and form a continuous line between the EIAV Recombinant p26 Antigen (Ag) well and sample well (S3, Figure 2).
2. Weak Positive - Positive Control Reference Serum (RS) lines bend slightly towards the EIAV Recombinant p26 Antigen (Ag) well and away from the Positive Control Reference Serum wells but do not form a complete line (S2, Figure 2).
3. Negative - Positive Control Reference Serum (RS) lines do not bend, or bend slightly away from the EIAV Recombinant p26 Antigen well (Ag), and continue into the sample well (S1, Figure 2).
4. Very Strong Positive - Positive Control Reference Serum (RS) lines turn in towards the EIAV Recombinant p26 Antigen (Ag) well and a broad, hazy line is formed closer to the Antigen Well than the sample well (S3, Figure 3).
5. Non-Specific Lines - These may occur between the EIAV Recombinant p26 Antigen well and sample well however, the Positive Control Reference Serum lines will pass through these lines. The non-specific lines do not form a continuous line with the Positive Control Reference Serum lines. A negative or EIAV-specific positive identity line may still be produced with the presence of non-specific lines however, care must be used to ensure the sample is interpreted according to the termination of control reference positive lines (S2, Figure 3).
6. Halo around the well - A halo or haze may occur around some sample wells caused by lipids or other materials in the serum. If the Positive Control Reference Serum lines can still be clearly read, the halo does not affect the results. However, if this halo makes reading the Positive Control Reference Serum lines difficult, a retest should be performed (S1, Figure 3).
Notes:
1. Read at 24-48 hours. Weak positives can take up to 48 hours to form; sera from suspect cases should be read again at 48 hours.
2. A positive result indicates the presence of precipitating antibodies against EIAV in the serum. A negative result indicates antibodies against EIAV were not detected.
3. All positives, including weak positives, should be retested prior to reporting results.
4. Questionable results should be retested prior to reporting. In the United States, samples continuing to produce questionable or doubtful results should be submitted to the National Veterinary Services Laboratories for confirmation. For other countries, users should follow the applicable laws and policies of their national animal health authorities.
5. If a dam is positive, the foal may test positive for more than 6 months, due to colostral antibody. In such a case, the foal and dam should be tested monthly up to 12 months of the foal’s age to determine if the foal becomes negative.
6. Once a horse is determined to be positive on this assay, it is considered infected for life.
7. Suspect horses (i.e. horses with clinical signs or a history suggesting exposure to EIAV) testing negative should be retested in 2-3 weeks.
8. EIAV infection may be acute, chronic or unapparent. Horses with any of these forms may test positive for antibody. Horses may test as weak positives during the incubation period. If exposure is suspected, a second sample should be tested after 2-3 weeks.
Sale and use of the test is restricted to laboratories approved by state and federal (USDA) authorities. Positive, discrepant or equivocal samples should be referred to the state or federal laboratories (NVSL) for confirmation and those outcomes reported to state officials immediately.
VLN 430/PCN 5515.22
For Animal Use Only
Manufactured by: SafePath Laboratories, LLC., Carlsbad, CA 92010 USA
760-929-7744
Distributed by: Centaur, Inc., 1351 Old 56 Highway West, Bldg. F, Olathe, KS 66061
800-236-6180 - www.centauranimalhealth.com
PN 4933 Rev. 1
CPN: 1488036.1
1351 W. HIGHWAY 56, BUILDING F, OLATHE, KS, 66061
| Telephone: | 913-390-6184 | |
| Order Desk: | 800-236-6180 | |
| Fax: | 913-390-5907 | |
| Website: | www.centauranimalhealth.com | |
| Email: | sales@centauranimalhealth.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
