Butatron Tablets
This treatment applies to the following species: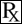 Company: Bimeda
Company: Bimeda
(phenylbutazone tablets, USP)
1 gram
Anti-Inflammatory
Butatron Tablets Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
Phenylbutazone is a non-hormonal, anti-inflammatory agent. Chemically, it is described as 4-Butyl-1,2-diphenyl-3,5 pyrazolidinedione, a synthetic pyrazolone derivative, entirely unrelated to the steroid compounds.
ACTIONS AND USES:
Kuzell(1,2,3), Payne(4), Flemming(5) and Denko(6), demonstrated clinical effectiveness of phenylbutazone in acute rheumatism, gout, gouty arthritis and various other rheumatoid diseases in man. Anti-inflammatory activity has been well established by Fabre(7), Domenjoz(8), Wilhelmi(9), and Yourish(10).
Lieberman(11) reported on the effective use of phenylbutazone in the treatment of conditions of the musculoskeletal system in dogs, including posterior paralysis associated with intervertebral disc syndrome, fractures, arthritis and injuries to the limbs and joints. Joshua(12) observed objective improvement without toxicity following long-term therapy of two aged arthritic dogs. Ogilvie and Sutter(13) reported rapid response to phenylbutazone therapy in a review of 19 clinical cases including posterior paralysis, posterior weakness, arthritis, rheumatism and other conditions associated with lameness and musculoskeletal weakness.
Camberos(14) reported favorable results with phenylbutazone following intermittent treatment of thoroughbred horses for arthritis and chronic arthrosis (e.g. osteoarthritis of medial and distal bones of the hock, arthritis of the stifle and hip, arthrosis of the spine and generalized arthritis). Results were less favorable in cases of traumatism, muscle rupture, strains and inflammatory conditions of the third phalanx. Sutter(15) reported favorable responses in chronic equine arthritis, fair results in a severely bruised mare, and poor results in two cases where the condition was limited to the third phalanx.
Butatron Tablets Indications
Phenylbutazone possesses non-hormonal, anti-inflammatory activity of value in the management of musculoskeletal conditions in dogs and horses such as the arthritides, including osteoarthritis, and as an aid in the relief of inflammation associated with intervertebral disc syndrome in dogs.
Contraindications
Phenylbutazone should not be administered to animals with serious hepatic, renal or cardiac pathology, or those with a history of blood dyscrasia.
Warning
PHENYLBUTAZONE SHOULD NOT BE ADMINISTERED TO MEAT, EGG OR MILK PRODUCING ANIMALS BECAUSE THE STATUS OF RESIDUES OF DRUG REMAINING IN EDIBLE TISSUES HAS NOT BEEN DETERMINED.
HAZARDS AND PRECAUTIONS:
1. Use with caution in animals with a history of drug allergy.
2. Stop medication at the first sign of gastrointestinal upset, jaundice or blood dyscrasia. Authenticated cases of agranulocytosis associated with phenylbutazone have occurred in man. Phenylbutazone induced blood dyscrasias have been reported in dogs. Thrombocytopenia and leukopenia are early manifestations followed by nonregenerative anemia. The occurrence of this reaction is not dose dependent and is unpredictable. To guard against this possibility, conduct routine blood counts at not more than 7 day intervals during the early course of therapy, and at intervals of not more than 14 days throughout the course of therapy. Any significant fall in the total white count, relative decrease in granulocytes or black or tarry stools should be regarded as a signal for immediate cessation of therapy and institution of appropriate treatment.
3. When treating inflammatory conditions associated with infection, specific anti-infective therapy is required.
4. Response to phenylbutazone therapy is prompt, usually occurring within 24 hours. If no significant clinical response is evident after 5 days of therapy, re-evaluate diagnosis and therapeutic regimen.
DOSAGE - DOGS:
Orally
20 mg per pound body weight (100 mg/5 lbs.) daily in 3 divided doses, not to exceed 800 mg daily regardless of body weight.
DOSAGE - HORSES:
Orally
1-2 grams per 500 lbs. body weight, not to exceed 4 grams daily.
ADMINISTRATION:
1. Use a relatively high dose for the first 48 hours, then reduce gradually to a maintenance dose. Maintain lowest dose capable of producing the desired clinical response.
2. In many cases tablets may be crushed and given with feed. Reduce the dosage as symptoms regress. In some cases treatment may be given only when symptoms appear with no need for continuous medication.
3. In animals, phenylbutazone is largely metabolized in 8 hours. It is recommended that a third of the daily dose be administered at 8 hour intervals.
4. Many chronic conditions will respond to phenylbutazone therapy but discontinuance of treatment may result in the recurrence of symptoms.
Contact Information:
The Safety Data Sheet (SDS) contains more detailed occupational safety information. To report suspected adverse drug events, for technical assistance or to obtain a copy of the SDS, contact Bimeda, Inc. at 1-888-524-6332. For additional information about reporting adverse drug experience for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
Storage
Store at controlled room temperature, 20°C - 25°C (68°F - 77°F), with excursions permitted to 15°C - 30°C (59°F - 86°F).
How Supplied
Butatron (phenylbutazone tablets, USP) are supplied in the following tablet concentrations and package sizes:
|
1 gram tablets |
Bottles of 100 tablets |
References
1. KuzeIl, W.C., Schaffarzick, R.W., Naugler, W.G., Mankle, E.A., A.M.A. Arch. Int. Med. 92:646,1953.
2. Kuzell, W.C., Schaffarzick, R.W., Brown, B., Mankle, E.A., Jour. Amer. Med. Assoc. 149:729,1952.
3. Kuzell, W.C., Schaffarzick, R.W., Cal. Med. 77:319,1952.
4. Payne, R.W., Shetlar, M.R., Farr, C., Hellbaum, A.A., and Ismael, W.K.T., J. Lab. Clin. Med. 45:331,1955.
5. Flemming, J. and Will, G., Ann. Rheumat. Dis. 12:95,1953.
6. Denko, C.W., RumI, D., Amer. Practit. 6:1865,1955.
7. Fabre, J. and Berger, A., Semaine Hop. (Paris) 31:87,1955.
8. Domenjoz, R., Theobald, W. and Morsdorf, K., Arzneimittel-Forsch. 5:488,1955.
9. WiIheImi, G. and Pulver, R., Arzneimittel-Forsch. 5:221,1955.
10. Yourish, N., Paton, B., Brodie, B.B. and Burns, J.J., A.M.A. Arch. Ophth. 53:264,1955.
11. Lieberman, L.L., Jour. A.V.M.A. 125:128,1954.
12. Joshua, J.O., Vet. Rec. 68:60 (Jan. 21), 1956.
13. Ogilvie, F.B. and Sutter, M.B., Vet. Med. 52:492-494,1957.
14. Camberos, H.R., Rev. Med. Vet. (Buenos Aires) 38:9,1956.
15. Sutter, M.D., Vet. Med. 58:83 (Feb.), 1958.
EACH TABLET CONTAINS:
|
Phenylbutazone U.S.P. |
1 gram |
Phenylbutazone is a systemically active anti-inflammatory drug.
NOT FOR USE IN HUMANS
KEEP OUT OF REACH OF CHILDREN
Butatron® is a Registered Trademark of Bimeda, Inc.
Approved by FDA under NADA # 044-756.
N.A. Corp. Address:
Bimeda, Inc., One Tower Lane, Oakbrook Terrace, IL 60181
Manufactured by:
Bimeda, Inc., Le Sueur, Mn 56058
www.bimeda.com
8BUT003 Rev. 01/22
|
Net Contents: |
|
|
|
100 Tablets |
1BUT001 |
8BUT001 Rev: 01/22 |
CPN: 1399052.9
Div. Cross Vetpharm Group, Ltd.
ONE TOWER LANE-SUITE 2250, OAKBROOK TERRACE, IL, 60181
| Telephone: | 630-928-0361 | |
| Fax: | 630-928-0362 | |
| Website: | www.bimedaus.com | |
| Email: | us-sales@bimeda.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
