Bioestrovet (Canada)
This treatment applies to the following species: Company: Vetoquinol
Company: Vetoquinol
Cloprostenol Injection Mfr. Std.
Sterile Synthetic Prostaglandin for Cattle
DIN 02458896
Description
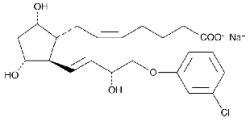
Bioestrovet cloprostenol is a synthetic prostaglandin analogue, structurally related to prostaglandin F2 (PGF2a).
Active Ingredients
Each 2 mL contains 500 µg of cloprostenol (as cloprostenol sodium).
PRESERVATIVES: Chlorocresol 0.1% w/v.
Non-medicinal Ingredients
Water for injection, sodium chloride, sodium citrate and citric acid.
Bioestrovet Indications
By its ability to shorten the life span of the corpus luteum Bioestrovet can be used to treat certain clinical conditions which delay breeding, to manipulate the estrous cycle to better fit certain management practices, and to induce abortion.
Therapeutic Indications
Sub-estrus (silent heat or non-detected estrus): Cows which fail to exhibit normal estrus behavior although ovarian cyclicity continues can be treated with Bioestrovet while in the luteal phase of the estrous cycle. They may then either be closely observed for estrus over a scheduled time period and bred on detection of estrus or bred at 72 and 96 hours after injection without estrus detection.
Pyometra or chronic endometritis: Damage to the reproductive tract at calving or post-partum retention of the placenta frequently leads to infection and inflammation of the uterus which is usually referred to as endometritis. Under certain circumstances, this may progress into chronic endometritis with the uterus becoming distended with purulent matter. This condition, frequently referred to as pyometra, is characterized by a lack of cyclical estrus behavior and the presence of a persistent corpus luteum. This condition can be successfully treated by causing regression of the C.L. by treatment with Bioestrovet. Where necessary, treatment may be repeated after 10 - 14 days.
Pregnancies from mis-mating and abortion in feedlot heifers: Unwanted pregnancies can be safely and efficiently terminated from one week after mating until about 4 1/2 months of gestation. The induced abortion is normally uncomplicated, the fetus and placenta are usually expelled about 4 or 5 days after the injection and the reproductive tract returns to normal soon after the abortion. Trial results have demonstrated that an abortion rate of approximately 95% can be expected up to 4 1/2 months of gestation. The ability of Bioestrovet to induce abortion decreases beyond 4 1/2 months while the risk of dystocia and its consequences increases.
Mummified fetus: Death of the conceptus during gestation may be followed by its degeneration and dehydration. Induction of luteolysis with Bioestrovet usually results in the expulsion of the mummified fetus from the uterus. (Manual assistance may be necessary to remove the fetus from the vagina. Normal cyclical activity should then follow.)
Controlled breeding: The luteolytic action of Bioestrovet can be used to schedule estrus and ovulation for an individual animal or a group of animals. This allows control of the time at which cycling cows or heifers can be bred.
Reproductive synchrony: Bioestrovet is indicated with a gonadorelin injectable solution* to synchronize estrous cycles to allow for fixed-time artificial insemination (FTAI) in lactating dairy cattle.
*For use only with gonadorelin injectable solutions comprised of 45 µg/mL gonadorelin acetate (equivalent to 43 µg gonadorelin) with a label indication for reproductive synchrony in cattle.
Bioestrovet Dosage And Administration
Bioestrovet should be administered by INTRAMUSCULAR INJECTION. Only cattle with a functional C.L. can respond to the luteolytic action of Bioestrovet. In the cycling animal there are refractory periods of 4 to 5 days before and after ovulation when cattle are not responding to prostaglandin.
Therapeutic indications: 2 mL
Abortion: 1.5 mL (2 mL for animals over 455 kg).
Controlled breeding: 2 mL
It is recommended that the vial is not broached more than 10 times and that the appropriate vial size used for prevailing usage conditions. Otherwise, automatic syringe equipment, or a suitable draw-off needle, should be used for the 50 ml and 100 ml vials to avoid excessive puncturing of the stopper.
Injection Regimens
For abortion and for therapeutic indications - one injection. For controlled breeding - Veterinarians and their client dairy and beef producers should select the controlled breeding program (A, B or C) which is appropriate for the existing circumstances and management practices.
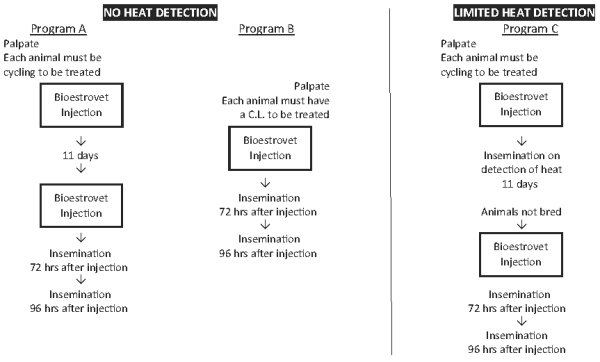
First service conception rates in Canada using existing artificial insemination practices are generally agreed to be between 40% and 60% for beef cattle and 50% to 60% for dairy cattle. The spread of calf crops indicates similar levels are attained using natural service. Field trial results have demonstrated that producers can achieve similar conception rates using cloprostenol. If existing management breeding practices are resulting in higher or lower conception rates, similar levels can be expected using Bioestrovet. Following the controlled breeding program those animals not conceiving should be rebred. This may be done by:
● Observing animals for a return to estrus (especially during the third week after injection) and inseminating or hand mating animals returning to estrus, or
● Turning in clean-up bull(s) 7 to 8 days after the last injection to cover any animal returning to estrus.
Many factors affect conception rates. Before a controlled breeding program is planned, the producer and his consulting veterinarian should review the operation’s breeding history, herd health and nutritional status and agree that a controlled breeding program is practical in the producer’s specific situation. For a successful controlled breeding program:
● Cows and heifers must be cycling. Cattle should be palpated.
● Cattle should be in good condition for breeding. Animals in poor or medium condition should be fed to ensure a positive nutritional balance for 4-6 weeks before Bioestrovet treatment and for 4 weeks after treatment.
● Proper program planning and record keeping are essential.
● Artificial insemination must be performed by competent inseminators using high quality semen. Inseminator fatigue must be avoided.
Reproductive synchrony:
For use with a gonadorelin injectable solution* to synchronize estrous cycles to allow FTAI: Administer the recommended intramuscular dosage of gonadorelin in reproductive synchrony programs similar to the following:
*For use only with gonadorelin injectable solutions comprised of 45 µg/mL gonadorelin acetate (equivalent to 43 µg gonadorelin) with a label indication for reproductive synchrony in cattle.
● Day 0: Administer the first gonadorelin injection
● Day 6-8: Administer 2 mL of Bioestrovet (500 µg cloprostenol)
● Day 8-11: Administer the second gonadorelin injection 30 to 72 hours after the Bioestrovet injection
● Day 9-12: Perform FTAI 8 to 24 hours after the second gonadorelin injection, or inseminate cows on detected estrous using standard herd practices.
Contraindications
Since Bioestrovet results in an abortion rate of approximately 95% in cattle up to 4 1/2 months of gestation and causes some cattle in later pregnancy to abort, it should not be given to pregnant animal unless induced abortion is desired.
Bioestrovet Cautions
To reduce the risk of anaerobic infections care should be taken to avoid injection through contaminated areas of skin. Clean and disinfect injection sites thoroughly before application.
Warnings
No meat withdrawal period and no milk withholding time are required when used according to the label. This product should be handled carefully to avoid accidental self-injection or contact with the skin or mucous membranes of the user.
Prostaglandins of the F2a type may readily be absorbed through the skin and may cause bronchospasms and/or miscarriage.
Pregnant women, women of childbearing age, asthmatics and people with other respiratory tract diseases should exercise extreme caution when handling this product such as wearing waterproof gloves.
Accidental spillage on the skin should be washed off immediately with water.
In case of accidental self-injection, seek medical advice and show the package insert to the doctor.
Should respiratory distress result from accidental inhalation or injection, the inhalation of a rapidly acting bronchodilator is indicated.
Keep out of the reach of children.
Adverse Reactions
As with other products in this class, when used for induction of parturition, the incidence of retained placenta may be increased depending on the time of treatment.
A low incidence of clostridial and other infections at the injection site has been reported following prostaglandin administration. Treated animals should be closely observed post injection and appropriate antibiotic therapy initiated at the first sign(s) of infection.
Very rarely, anaphylactic reactions have occurred after the administration of the product.
Overdose: At 50 and 100 times the recommended dose of cloprostenol mild side effects may be detected. These included increased uneasiness, mild transient diarrhea, slight frothing and milk letdown.
Action
Bioestrovet causes functional and morphological regression of the corpus luteum in cattle. This effect on the life span of the C.L. usually results in estrus two to five days after treatment, followed by ovulation with normal fertility. Bioestrovet alone will not increase fertility.
Storage Conditions
Store between 15°C and 25°C. Protect from light and store in product carton.
Contents should be used within 28 days after the first dose is removed.
HOW SUPPLIED
20 mL, 50 mL and 100 mL multidose vials.
Vetoquinol N.-A. Inc., 2000, ch. Georges, Lavaltrie, QC J5T 3S5
449304 0217 A
|
Net |
Code |
|
20 mL |
443860 |
|
50 mL |
443863 |
|
100 mL |
443864 |
CPN: 1234434.1
Commercial Division
2000, CHEMIN GEORGES, LAVALTRIE, QC, J5T 3S5
| Telephone: | 450-586-2252 | |
| Order Desk: | 800-363-1700 | |
| Fax: | 450-586-4649 | |
| Website: | www.vetoquinol.ca | |
| Email: | info@vetoquinol.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
