Bimeprazol (Canada)
This treatment applies to the following species: Company: Bimeda-MTC
Company: Bimeda-MTC
(omeprazole oral paste 37% w/w)
DIN 02531674
For the Treatment and Prevention of Equine Gastric Ulcers
FOR VETERINARY USE ONLY
Oral Paste for Horses and Foals
Description
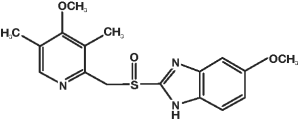
● Chemical name: 5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole.
Empirical formula: C17H19N3O3S.
Molecular weight: 345.42.
Structural formula:
Bimeprazol Indications
● To aid in improving, healing and preventing the occurrence and recurrence of gastric ulcers, in horses and foals nine weeks of age and older.
DOSAGE:
● For the treatment of gastric ulcers: 4 mg omeprazole per kg body weight, once daily orally for 28 consecutive days (4 weeks).
● To aid in the prevention of the recurrence of gastric ulcers: continue treatment at a dosage regimen of 2 mg omeprazole per kg body weight, once daily orally for 28 additional days.
● To aid in the prevention of occurrence of gastric ulcers: 1 mg omeprazole per kg body weight, once daily orally for up to 28 days or as prescribed by a veterinarian.
Directions For Use
Bimeprazol paste is recommended for use in horses and foals nine weeks of age and older.
● To deliver the product at the treatment dosage rate of 4 mg omeprazole/kg, set the syringe plunger to the appropriate weight marking according to horse’s weight in kg. The contents of one syringe will treat a 575 kg horse at the rate of 4 mg omeprazole per kg body weight.
● To deliver the product at the dosage rate of 2 mg omeprazole/kg body weight to aid in the prevention of recurrence of gastric ulcers, set the syringe plunger to the weight marking corresponding to half of the horse’s weight in kg. For example, to treat a horse weighing 500 kg, set the plunger to 250 kg.
● To deliver the product at the preventative dosage rate of 1 mg omeprazole/kg, set the syringe plunger to the weight marking corresponding to one quarter the horse’s body weight in kg. For example, to treat a horse weighing 400 kg, set the plunger to 100 kg.
● Remove the plastic cap from the syringe tip. Make sure the horse’s mouth contains no feed. Insert the syringe into the horse’s mouth at the interdental space. Depress the plunger until stopped by the knurled ring. The dose should be deposited on the back of the tongue or deep into the cheek pouch. Care should be taken to ensure that the horse consumes the complete dose. Treated animals should be observed briefly after administration to ensure that part of the dose is not lost or rejected. If any of the dose is lost, redosing is recommended.
● If, after dosing, the syringe is not completely empty, it may be re-used on following days until emptied. Replace the cap after each use.
CAUTIONS:
● The safety of omeprazole has not been determined in breeding, pregnant or lactating mares.
● Omeprazole is metabolized by the cytochrome P450 system, mainly in the liver. In humans, prolonged elimination of drugs metabolized through the cytochrome P450 system, including benzodiazepines, occurs with concomitant use of omeprazole. The clinical significance of these findings in horses have not been investigated. Horses with hepatic disease or a history of hepatic disease should be carefully monitored.
Warnings
● This drug is not to be administered to horses that are to be slaughtered for use in food. In case of ingestion, contact a physician. Physicians may contact a poison control center for advice concerning accidental ingestion. Keep out of reach of children.
Adverse Reactions
● In efficacy trials conducted with omeprazole paste, no adverse events were observed.
Clinical Pharmacology
Mechanism of action:
Omeprazole is a gastric acid pump inhibitor that regulates the final step in hydrogen ion production and blocks gastric acid secretion regardless of the stimulus. Omeprazole irreversibly binds to the gastric parietal cell’s H+, K+ ATPase enzyme which pumps hydrogen ions into the lumen of the stomach in exchange for potassium ions. Since omeprazole accumulates in the cell cannaliculi and is irreversibly bound to the effect site, the plasma concentration at steady state is not directly related to the amount that is bound to the enzyme. The relationship between omeprazole action and plasma concentration is a function of the rate-limiting process of H+, K+ ATPase activity/turnover. Once all of the enzyme becomes bound, acid secretion resumes only after new H+, K+ ATPase is synthesized in the parietal cell (i.e., the rate of new enzyme synthesis exceeds the rate of inhibition).
Pharmacodynamics:
At 8, 16 and 24 hours after dosing horses with omeprazole at 4 mg/kg/day orally, pentagastrin-stimulated gastric acid secretion was inhibited by 99%, 95% and 90%. The full effect on the inhibition of acid secretion is reached by five days after the first administration.
Pharmacokinetics:
The median bioavailability of omeprazole after oral administration as a paste is 10.5% (range 4.1 to 12.7%).1 A comparative bioavailability study was performed using 32 healthy adult horses. The rate and extent of absorption of omeprazole were measured and compared following administration of a 4 mg/kg dose of either an oral suspension of omeprazole or Bimeprazol under fasting conditions. Results from this study showed that the drug absorption is rapid with time to maximum plasma concentrations (Tmax) 0.5 to 2 hours after dosing. Mean peak concentration (Cmax) ranges from 183 ng/ml to 668 ng/ml after dosing with 4 mg/kg bw. There is a significant first-pass effect following oral administration. Omeprazole is rapidly metabolized principally into glucuronides of demethylated and hydroxylated omeprazole sulphide (urinary metabolites) and methyl sulphide omeprazole (biliary metabolite) as well as into reduced omeprazole (both). After oral administration at 4 mg/kg bw, omeprazole is detectable in plasma for 6 hours after treatment, and in urine as hydroxyomeprazole and O-desmethylomeprazole at 24 hours but not at 48 hours. Omeprazole is eliminated quickly, mainly by urinary route (43 to 61% of the dose), and to a smaller extent by fecal route, with a terminal half-life ranging from approximately 0.5 to 2.05 hours. After repeated oral administration, there is no evidence of accumulation.1
[1. CVMP Omeprazole Summary Report (EMEA/MRL/841/02-FINAL, June 2002)]
SAFETY:
No adverse effects related to treatment were observed following daily use of omeprazole at dosages up to 20 mg/kg in adult horses for 90 days and in foals older than 2 months for 91 days.
No adverse effects related to treatment (in particular no adverse effect on the semen quality or reproductive behaviour) were observed following daily use of omeprazole at a dosage of 12 mg/kg in breeding stallions for 70 days.
No adverse effects related to treatment were observed following daily use of omeprazole at a dosage of 40 mg/kg in adult horses for 21 days.
EFFICACY:
● Omeprazole paste treatment at 4 mg/kg daily for 28 days has been shown to effectively heal or reduce the severity of gastric ulcers. Subsequent daily administration of omeprazole paste at 2 mg/kg for 30 days prevented recurrence of gastric ulcers. Additionally, administration of omeprazole paste at 1 mg/kg daily to healthy horses confirmed not to have gastric ulcers, effectively prevented the occurrence of gastric ulcers when horses were submitted to ulcerogenic conditions.
● Diagnostic and Management Considerations: The following clinical signs may be associated with gastric ulceration in adult horses: inappetence or decreased appetite, recurrent colic, intermittent loose stools or chronic diarrhea, poor hair coat, poor body condition, or poor performance. Clinical signs in foals may include: bruxism (grinding of teeth), excess salivation, colic, cranial abdominal tenderness, anorexia, diarrhea, sternal recumbency or weakness. A more accurate diagnosis of gastric ulceration in horses and foals may be made if gastric ulcers are visualized directly by gastroscopic examination of the gastric mucosa. Gastric ulcers may recur in horses if therapy to prevent recurrence is not administered after the initial treatment is completed. Use Bimeprazol paste at 2 mg omeprazole/kg body weight for the prevention of gastric ulcer recurrence following treatment, and at 1 mg omeprazole/kg body weight for the prevention of occurrence in horses with no recent history of gastric ulcers. The safety of administration of omeprazole paste for longer than 91 days has not been determined. Maximal acid suppression occurs after three to five days of treatment with omeprazole. Consult your veterinarian for the diagnosis, treatment and control of gastric ulcers.
Storage
● Store between 15°C and 25°C; excursions permitted to 30°C. Use within 28 days of first opening. Do not freeze.
How Supplied
● Bimeprazol (omeprazole) paste for horses contains 37% w/w omeprazole and is available in an adjustable-dose syringe. Each syringe contains 2.28 g of omeprazole. Syringes are calibrated according to bodyweight and are available in boxes of 7 and 72 units.
FOR MORE INFORMATION:
Please call 1-877-456-2755
Bimeda-MTC Animal Health Inc., Cambridge, ON N3C 2W4
Distributed by Solvet
8OME003
Rev 07/23
|
Net: |
|
|
|
Contains 7 syringes, 6.16 g |
1OME007 |
8OME005 Rev 07/23 8OME001 Rev 07/23 |
|
Contains 72 syringes, 6.16 g |
1OME008 |
8OME004 Rev 07/23 8OME001 Rev 07/23 |
CPN: 1989001.0
Distributed by SOLVET
7226-107th AVENUE S.E., CALGARY, AB, T2C 5N6
| Téléphone: | 403-456-2245 | |
| Contactez-nous sans frais au: | 877-456-2755 | |
| Fax: | 403-271-2920 | |
| Site Web: | www.solvet.ca | |
| Courriel: | info@solvet.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
