Baycox (toltrazuril) 5% Oral Suspension (Canada)
This treatment applies to the following species: Company: Elanco
Company: Elanco
5% Toltrazuril
Oral Suspension Prof. Std.
VETERINARY USE ONLY
DIN 02355353
Active Ingredient
toltrazuril
Medicinal ingredient per mL:
50 mg toltrazuril
Baycox (toltrazuril) 5% Oral Suspension Indications
Baycox is indicated for the treatment of preclinical coccidiosis due to Isospora suis in neonatal piglets, for the prevention of clinical signs of coccidiosis and reduction of coccidian shedding in lambs on farms with a confirmed history of coccidiosis caused by Eimeria crandallis and Eimeria ovinoidalis, and for the prevention of clinical signs of coccidiosis and reduction of coccidian shedding in calves on farms with a confirmed history of coccidiosis caused by Eimeria bovis and Eimeria zuernii.
Baycox is not indicated for use in feedlot cattle.
Dosage and Administration
Piglets: Shake well before use. Weigh three representative litters at 3 days of age to determine an average piglet weight. The recommended dosage of toltrazuril is 20 mg per kg body weight. This corresponds to a dose of 1 mL Baycox per 2.5 kg body weight.
Administer a single oral dose at 3 to 4 days of age.
Lambs: Shake well before use. Each animal should be treated with a single dose of 20 mg toltrazuril/kg body weight corresponding to 1 mL Baycox per 2.5 kg body weight. To obtain maximum benefit, sheep should be treated in the prepatent period before the expected onset of clinical signs. The prepatent period of Eimeria ovinoidalis is 12-15 days and the prepatent period of Eimeria crandalis is 15-20 days. If animals are to be treated collectively rather than individually, they should be grouped according to their body weight and dosed accordingly, in order to avoid under- or overdosing. In order to maximize effectiveness of Baycox in lambs it is important to time therapy according to individual farm management and lifecycle of the organism involved.
Calves: Shake well before use. Each animal should be treated with a single oral dose of 15 mg toltrazuril/kg body weight corresponding to 3 mL Baycox per 10 kg body weight. To obtain maximum benefit, calves should be treated in the prepatent period before the expected onset of clinical signs. The prepatent period of Eimeria zuernii is 15-17 days and the prepatent period of Eimeria bovis is 18-21 days. If animals are to be treated collectively rather than individually, they should be grouped according to their body weight and dosed accordingly, in order to avoid under- or overdosing. It is important to time therapy according to individual farm management and lifecycle of the organism involved.
Cautions:
As with any antiparasiticide, frequent and repeated use of antiprotozoals from the same class may lead to the development of resistance.
Warnings
Piglets: Treated swine must not be slaughtered for use in food for at least 70 days after the latest treatment with this drug. Do not use in piglets intended to be used as suckling or barbecue pigs since they may be marketed before 70 days after administration of this drug. Lambs: Treated sheep must not be slaughtered for use in food for at least 48 days after the latest treatment with this drug.
- Do not use in lactating sheep producing milk for human consumption.
Calves: Treated cattle must not be slaughtered for use in food for at least 63 days after the latest treatment with this drug.
- Do not use in calves to be processed for veal. A withdrawal period has not been established for this product in pre-ruminating calves.
- Do not use in lactating dairy cows producing milk for human consumption.
Avoid direct contact with skin by wearing rubber gloves while handling this drug. Dispose the unused drug in accordance with the Provincial/Municipal guidelines.
Keep out of reach of children
Adverse Reactions
Although all adverse reactions are not reported, the following adverse reaction information is based on voluntary post-approval drug experience reporting. It is generally recognized that this method of reporting results in significant under-reporting of adverse drug reactions. It should be noted that suspected adverse drug reactions listed here reflect reporting and not causality. The categories of adverse reactions are listed in decreasing order of frequency by body system.
Lack of efficacy has been reported in countries other than Canada. To reduce concerns associated with lack of efficacy, follow label instructions regarding correct dose and timing.
Studies indicate that the metabolite, toltrazuril sulfone, accumulates in soil when undiluted manure from treated feedlot cattle is repeatedly spread on the same agricultural field. This may affect groundwater and negatively impact the growth of certain crop species.
To report suspected adverse drug events or for technical assistance, contact Elanco Canada Limited at 1-800-265-5475.
Pharmacology
Chemical Name: 1-[3-methyl-4- (4’-trifluoromethylthiophenoxy)-phenyl]-3 methyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione
Molecular formula: C18H14F3N304S
Structural formula:
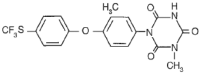
Molecular weight: 425.38
Mode of Action: Light and electron microscope studies show that toltrazuril is active against all intracellular stages of coccidia, including schizonts, micro and macrogamonts. It interferes with the division of the protozoal nucleus, the activity of the mitochondria and damages the wall forming bodies in the microgametes. Toltrazuril produces severe vacuolisation of the protozoal endoplasmic reticulum in all intracellular development stages.
Pharmacodynamics, Pharmacokinetics: After oral administration, toltrazuril is absorbed slowly from the gut, indicated by a radio-active study using [triazine-2-14C]-marked toltrazuril. This is followed by a long-lasting distribution among the different compartments of the body. The plasma half-life is 51 hours in piglets, about 170 hours in lambs, and about 72 hours in calves. Excretion is characterized by a high fecal fraction with a relatively high excretion rate. There is no significant enterohepatic circulation.
Two metabolites of toltrazuril, both oxidation products, toltrazuril-sulfoxide and toltrazuril-sulfone, are found in the tissues and organs of piglets, lambs, and calves.
Efficacy:
Piglets: The efficacy of a single oral treatment with toltrazuril against preclinical neonatal coccidiosis in pigs has been widely demonstrated by several investigators in countries as diverse as Australia, Canada, Denmark, Italy, Malaysia and Venezuela, under various conditions of management and hygiene. Treated piglets showed improvement regarding their clinical picture (diarrhea was the most frequently noted criterion), oocyst shedding, and had better weight gains than the untreated animals.
Lambs: Naturally infected lambs were treated with toltrazuril 20 mg/kg body weight 10 days after turn out onto pasture. The oocyst excretion was observed up until 9 weeks of age vs an untreated control group for 5 weeks. The metaphylactic efficacy of toltrazuril was studied in Norway. Seven to 9 days after turnout on pasture, lambs were treated with toltrazuril at 20 mg/kg to prevent coccidiosis. Treatment with Baycox reduced the oocytes output significantly and prevented the development of diarrhea during the first 4-5 weeks after treatment. Treated animals had better weight gains than untreated animals.
Calves: Naturally and artificially infected calves in multiple studies received treatment with Baycox at 15 mg/kg body weight. Calves treated metaphylactically during the prepatent period showed significant reduction in oocyst shedding, and a reduction in the severity and duration of diarrhea. Treated animals had better weight gains than the untreated animals.
Safety:
Piglets: The tolerance of neonatal pigs to Baycox was tested extensively under controlled conditions and assessed on the basis of clinical, haematological and clinicochemical parameters, which were determined before and after treatment and by comparison with untreated controls. Animals were also examined for gross pathological and histological changes.
Studies performed with single treatments of 20 mg/kg (recommended rate), 60 mg/kg (3 x recommended), 100 mg/kg (5 x recommended) and at 30 mg/kg once a day for 2 days showed that the formulation is well tolerated at the therapeutic dosage:
● there was no influence on body weight development in any of the groups,
● the haematological and clinical-chemical investigations revealed no changes related to treatment,
● the gross pathology studies showed no indication of change to the animals or their organs.
Lambs: A tolerability study was performed in 1-3 week old lambs. Four groups of lambs were treated with 0 - 20 - 60 - 2x40 mg/kg body weight. The last group was treated twice on 2 consecutive days. Clinical examinations as well as hematology, clinical chemistry, body weight development, feed intake and gross and histopathology were performed. No compound related effects were seen in any of the groups dosed with toltrazuril.
Calves: Safety was demonstrated in one pivotal trial involving 30 animals aged between 2 and 4 months. Untreated control animals, animals dosed at 15 mg/kg (label dose) and animals dosed at 45 mg/kg (3X label dose) were observed for 90 days at which time post mortem examinations were done. The product was well tolerated by both treatment groups. Mild elevations of bilirubin were seen in the 3X (45mg/kg) group which returned to normal after 4 weeks.
Storage
Store below 30°C. Protect from freezing.Baycox (toltrazuril) 5% Oral Suspension is available in 250 mL and 1 L bottles.
Elanco Canada Limited, 1919 Minnesota Court, Suite 401, Mississauga, Ontario L5N 0C9
Baycox is sold by Elanco or its affiliates and is not a product of Bayer. The Product Name Baycox is owned by Bayer and used under license.
© 2022 Elanco or its affiliates.
03May2022
CPN: 1231199.1
1919 MINNESOTA COURT, SUITE 401, MISSISSAUGA, ON, L5N 0C9
| Customer Service: | 800-265-5475 | |
| Fax: | 519-821-7831 | |
| Website: | www.elanco.ca | |
| Email: | elancocanadacustomerservice@elancoah.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
