Atipam (Canada)
This treatment applies to the following species: Company: Dechra
Company: Dechra
Atipamezole Hydrochloride Injection
Veterinary Use Only
Sterile - 5.0 mg/mL
Dexmedetomidine and Medetomidine Reversing Agent
DIN 02491710
Description
Atipam (atipamezole hydrochloride) is a synthetic α2-adrenergic antagonist which reverses the effects of dexmedetomidine hydrochloride and medetomidine hydrochloride in dogs. The chemical name is 4-(2-ethyl-2,3-dihydro-1H-inden-2-yl)-1H-imidazole hydrochloride. The molecular formula is C14H16N2 • HCl and the structural formula is:
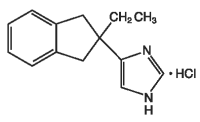
Each mL of Atipam contains 5.0 mg atipamezole hydrochloride as the medicinal ingredient, 1.0 mg methyl parahydroxybenzoate as the preservative, 8.5 mg sodium chloride, and water for injection.
Atipam Indications
Atipam is indicated for the reversal of the clinical effects of the sedative and analgesic agents, dexmedetomidine hydrochloride and medetomidine hydrochloride, in dogs.
Dosage and Administration
Atipam is administered intramuscularly regardless of the route used for dexmedetomidine or medetomidine. The concentration of Atipam has been formulated such that the volume of injection is the same (mL for mL) as the recommended dose volume of dexmedetomidine hydrochloride sterile injectable solution or medetomidine hydrochloride sterile injectable solution, and may be given at any time following dexmedetomidine hydrochloride sterile injectable solution or medetomidine hydrochloride sterile injectable solution administration. Although injection volumes are the same, the concentration of Atipam (5.0 mg/mL) is 10 times that of dexmedetomidine hydrochloride sterile injectable solution (0.5 mg/mL) and 5 times that of medetomidine hydrochloride sterile injectable solution (1.0 mg/mL). Dogs that are sedated but ambulatory may be treated with Atipam, if warranted.The dosage of Atipam is calculated based upon body surface area. Use the following tables to determine the proper injection volume based on bodyweight. Note that the mcg/kg dosage decreases as body weight increases.
Table 1: Atipamezole dosing for reversal of IV dexmedetomidine- or medetomidine-induced sedation/analgesia:
|
Dose table for Atipam when dexmedetomidine or medetomidine is given IV |
||
|
Bodyweight (kg) |
Dose = mcg/kg |
Volume = mL Atipam |
|
2-2.9 |
300 |
0.1 |
|
3-3.9 |
250 |
0.15 |
|
4-4.9 |
230 |
0.2 |
|
5-9.9 |
200 |
0.3 |
|
10-14.9 |
170 |
0.4 |
|
15-19.9 |
150 |
0.5 |
|
20-24.9 |
140 |
0.6 |
|
25-29.9 |
130 |
0.7 |
|
30-36.9 |
120 |
0.8 |
|
37-44.9 |
110 |
0.9 |
|
45-49.9 |
105 |
1.0 |
|
50-59.9 |
100 |
1.1 |
|
60-64.9 |
95 |
1.2 |
|
65-74.9 |
93 |
1.3 |
|
75-79.9 |
91 |
1.4 |
|
>80 |
90 |
1.5 |
Table 2: Atipamezole dosing for reversal of IM dexmedetomidine- or medetomidine-induced sedation/analgesia:
|
Dose table for Atipam when dexmedetomidine or medetomidine is given IM |
||
|
Bodyweight (kg) |
Dose = mcg/kg |
Volume = mL Atipam |
|
2-2.9 |
400 |
0.15 |
|
3-3.9 |
350 |
0.2 |
|
4-4.9 |
300 |
0.3 |
|
5-9.9 |
250 |
0.4 |
|
10-12.9 |
230 |
0.5 |
|
13-14.9 |
210 |
0.6 |
|
15-19.9 |
200 |
0.7 |
|
20-24.9 |
180 |
0.8 |
|
25-29.9 |
170 |
0.9 |
|
30-32.9 |
160 |
1.0 |
|
33-36.9 |
150 |
1.1 |
|
37-44.9 |
145 |
1.2 |
|
45-49.9 |
140 |
1.3 |
|
50-54.9 |
135 |
1.4 |
|
55-59.9 |
130 |
1.5 |
|
60-64.9 |
128 |
1.6 |
|
65-69.9 |
125 |
1.7 |
|
70-79.9 |
123 |
1.8 |
|
>80 |
120 |
1.9 |
CAUTIONS: Atipam can produce an abrupt reversal of sedation and, presumably, analgesia. The potential for apprehensive or aggressive behavior should be considered in the handling of dogs emerging from sedation, especially those individuals who are likely to be in pain.
Information on use of atipamezole with concurrent drugs is inadequate, therefore caution should be exercised when administering multiple drugs. Animals should be monitored closely, particularly for persistent hypothermia, bradycardia, and depressed respiration, until the animal has recovered completely. Caution should be used in administration of anesthetic agents to elderly or debilitated animals.
While atipamezole does reverse the clinical signs associated with dexmedetomidine or medetomidine sedation, complete physiologic return to pretreatment status may not be immediate and should be monitored.
Atipamezole has not been evaluated in breeding animals; therefore, the drug is not recommended for use in pregnant or lactating animals, or in animals intended for breeding.
Warnings
Keep out of reach of children. Care should be taken to assure that Atipam is not inadvertently ingested as safety studies have indicated that the drug is absorbed when administered orally. As with all injectable drugs causing profound physiological effects, routine precautions should be employed when handling and using filled syringes, including washing eye and skin areas affected by accidental spillage. In case of accidental human exposure, a physician should be contacted.Adverse Reactions
Occasional vomiting may occur. Rarely, a brief state of excitement or apprehensiveness may be seen in treated dogs. Other potential side effects of α2-antagonists include hypersalivation, diarrhea, and tremors.OVERDOSE: Atipamezole was tolerated in healthy dogs receiving doses 10-fold the recommended dose and in dogs receiving repeated doses at 1-, 3-, and 5-fold doses, in the absence of an α2-antagonist. Signs of overdose were dose-related and consistent with those expected in non-sedated dogs having received a stimulant. Signs seen at elevated doses included excitement, panting, trembling, vomiting, soft or liquid feces or vasodilation (injection) of the sclera. Some localized skeletal muscle injury was seen at the injection site; but no associated clinical signs or complications were observed. Dogs receiving the proper dose in the absence of dexmedetomidine or medetomidine, or 3-fold overdose after dexmedetomidine or medetomidine sedation, exhibited no significant clinical signs.
Clinical Pharmacology
Activation of peripheral and central α2-adrenergic receptors is known to induce a pattern of pharmacological responses including sedation, reduction of anxiety, analgesia, bradycardia, and transient hypertension with a subsequently reduced blood pressure. Atipamezole is a potent α2-antagonist which selectively and competitively inhibits α2-adrenergic receptors. The result of atipamezole administration in the dog is the rapid recovery from the sedation and other clinical effects produced by the α2-adrenergic agonists, dexmedetomidine and medetomidine. Atipamezole is not expected to reverse the effects of other classes of sedatives, anesthetics, or analgesics.Rapid absorption occurs following intramuscular injection, with a maximum serum concentration reached in approximately 10 minutes. Onset of arousal is usually apparent within 5 to 10 minutes of injection, depending on the depth and duration of dexmedetomidine- or medetomidine-induced sedation. Elimination half-life from serum is less than 3 hours. Atipamezole undergoes extensive hepatic biotransformation, with excretion of metabolites primarily in urine.
A transient, approximately 10%, decrease in systolic blood pressure occurs immediately after administration of atipamezole to dexmedetomidine- or medetomidine-sedated dogs, followed by an increase in pressure within 10 minutes to the pre-atipamezole level. This is the opposite of the response to α2-agonist therapy, and is probably due to peripheral vasodilatation.
Atipamezole will produce a rapid improvement in dexmedetomidine- or medetomidine-induced bradycardia. An increase in heart rate is usually apparent within approximately 3 minutes of injection, but approximately 40% of dogs are not expected to immediately return to pre-sedative rate. Some dogs may experience brief heart rate elevations above-baseline. Respiratory rate also increases following atipamezole injection.
Storage
Store at room temperature between 15°C and 30°C. Do not freeze. Discard any remaining product after 28 days of opening the vial.PRESENTATION: 10 mL glass vials.
Eurovet Animal Health B.V., Handelsweg 25, 5531 AE Bladel, Netherlands
Distributed by:
Dechra Veterinary Products Inc., 1 Holiday Ave, East Tower, Suite 345, Pointe-Claire, Quebec, H9R 5N3, Canada
617486
CPN: 1786064.0
1 HOLIDAY AVE., EAST TOWER SUITE 345, POINT-CLAIRE, QC, H9R 5N3
| Toll-Free: | 855-332-9334 | |
| Technical Services: | 855-332-9334 Option 1 | |
| Technical Services Email: | technical.ca@dechra.com | |
| Website: | www.dechra.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
