Apoquel 3.6 mg chewable tablets (Canada)
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
DIN 02530651
DIN 02530678
DIN 02530686
oclacitinib chewable tablets
3.6 mg, 5.4 mg, 16 mg
Veterinary Use Only
Janus Kinase Inhibitor for dogs
Description
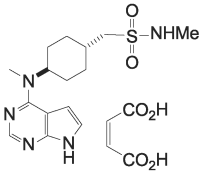
The active ingredient in APOQUEL™ chewable tablets is oclacitinib (as oclacitinib maleate), a synthetic Janus Kinase inhibitor. The chemical name of oclacitinib is N-methyl[trans-4-(methyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamino) cyclohexyl]methanesulfonamide (2Z)-2-butenedioate.
The chemical structure of oclacitinib maleate is:
Apoquel 3.6 mg chewable tablets Indications
For the control of pruritus associated with allergic dermatitis and for the control of atopic dermatitis in dogs at least 12 months of age.
Dosage and Administration
The dose of APOQUEL chewable tablets is 0.4 mg/kg to 0.6 mg oclacitinib/kg body weight, administered orally, twice daily for up to 14 days, and then administered once daily for maintenance therapy. APOQUEL chewable tablets are palatable and readily consumed by the majority of dogs (see EFFICACY). APOQUEL chewable tablets may be administered by hand, in a bowl, with or without food.Dosing Chart
|
Dog body weight (kg) |
3.6 mg chewable tablets |
5.4 mg chewable tablets |
16 mg chewable tablets |
|
3 - 4.4 |
0.5 |
|
|
|
4.5 - 5.9 |
|
0.5 |
|
|
6 - 8.9 |
1 |
|
|
|
9 - 13.4 |
|
1 |
|
|
13.5 - 19.9 |
|
|
0.5 |
|
20 - 26.9 |
|
2 |
|
|
27 - 39.9 |
|
|
1 |
|
40 - 54.9 |
|
|
1.5 |
|
55 - 80 |
|
|
2 |
Contraindications
APOQUEL chewable tablets should not be given to dogs with known or suspected allergy or intolerance to oclacitinib maleate or any other components of this product.
APOQUEL chewable tablets are not for use in dogs less than 12 months of age (see ANIMAL SAFETY).
APOQUEL chewable tablets are not for use in dogs with serious infections, evidence of immune suppression or evidence of malignant neoplasia.
APOQUEL chewable tablets are not for use in breeding dogs, pregnant or lactating bitches.
Cautions:
The safe use of oclacitinib has not been evaluated in dogs less than 3 kg (6.6 lbs) body weight.
The use of oclacitinib has not been evaluated in combination with glucocorticoids, cyclosporine, or other immunosuppressive agents.
APOQUEL chewable tablets modulate the immune system. Active infections should have resolved before starting therapy with oclacitinib.
APOQUEL chewable tablets may increase susceptibility to infection and development or exacerbation of neoplastic conditions (see ADVERSE REACTIONS). Therefore, dogs receiving APOQUEL chewable tablets should be monitored for signs of infection, including demodicosis, and neoplasia.
Consider the risks and benefits of treatment with APOQUEL chewable tablets prior to initiating therapy in dogs with a history of neoplasia.
Given the potential for effects on certain clinical pathology parameters, periodic monitoring with complete blood counts and serum biochemistry is recommended when dogs are on treatment long-term.
Warnings: Keep out of reach of children. Wash hands immediately after handling the chewable tablets. In case of accidental ingestion, seek medical attention immediately and show the package leaflet to the physician.
Adverse Reactions:
Although all adverse reactions are not reported, the following information is based on voluntary post-approval drug experience reporting. It is generally recognized that this results in significant under-reporting. The adverse events listed here reflect reporting and not necessarily causality.
Adverse events are listed, in decreasing order of frequency: Lack of efficacy, lethargy, vomiting, elevated liver enzymes, diarrhea, benign and malignant or unclassified neoplasms, including dermal masses (e.g. papillomas and histiocytomas), lymphoma and other neoplasia, anorexia, death (including euthanasia), anemia, and convulsion.
FIELD STUDY DATA
Control of Pruritus Associated with Allergic Dermatitis
In a masked field study to assess the efficacy and safety of oclacitinib for the control of pruritus associated with allergic dermatitis in dogs, 216 dogs treated with oclacitinib and 220 dogs treated with placebo (vehicle control) were evaluated for safety. During the 30-day study, there were no fatalities and no adverse reactions requiring hospital care. Adverse reactions reported (and percent of dogs affected) are found in Table 1.
Table 1: Adverse reactions observed in pruritus study up to day 7
|
|
Oclacitinib (n=216) |
Placebo (n=220) |
|
Diarrhea |
2.3% |
0.9% |
|
Vomiting |
2.3% |
1.8% |
|
Anorexia |
1.4% |
0% |
|
New cutaneous or subcutaneous lumps |
1.0% |
0% |
|
Lethargy |
1.8% |
1.4% |
|
Polydipsia |
1.4% |
0% |
In most of these cases, signs spontaneously resolved with continued dosing.
Five dogs in the oclacitinib group were withdrawn from the study because of: darkening areas of skin and fur (1 dog); diarrhea (1 dog); fever, lethargy and cystitis (1 dog); an inflamed footpad and vomiting (1 dog); and diarrhea, vomiting, and lethargy (1 dog).
Dogs in the oclacitinib group had a slight decrease in mean white blood cell counts (neutrophil, eosinophil, and monocyte counts) that remained within the normal reference range. Mean lymphocyte count for dogs in the oclacitinib group increased at Day 7 but returned to pre-treatment levels by study end without a break in APOQUEL administration. Serum cholesterol increased in 25% of dogs in the oclacitinib group, but mean cholesterol remained within the reference range.
Control of Atopic Dermatitis
In a masked field study to assess the efficacy and safety of oclacitinib for the control of atopic dermatitis in dogs, 152 dogs treated with oclacitinib and 147 dogs treated with placebo (vehicle control) were evaluated for safety. The majority of dogs in the placebo group withdrew from the 112-day study by Day 16. Adverse reactions reported (and percent of dogs affected) are found in Table 2.
Table 2: Adverse reactions observed in atopic dermatitis study up to day 16
|
|
Oclacitinib (n=152) |
Placebo (n=147) |
|
Diarrhea |
4.6% |
3.4% |
|
Vomiting |
3.9% |
4.1% |
|
Anorexia |
2.6% |
0% |
|
New cutaneous or subcutaneous lumps |
2.6% |
2.7% |
|
Lethargy |
2.0% |
1.4% |
|
Polydipsia |
0.7% |
1.4% |
In most cases, diarrhea, vomiting, anorexia, and lethargy spontaneously resolved with continued dosing.
Dogs on oclacitinib had decreased leukocytes (neutrophil, eosinophil, and monocyte counts) and serum globulin, and increased cholesterol and lipase compared to the placebo group, but group means remained within the normal range. Mean lymphocyte counts were transiently increased at Day 14 in the oclacitinib group.
Dogs that withdrew from the masked field study could enter an unmasked study where all dogs received oclacitinib. Between the masked and unmasked study, 283 dogs received at least one dose of oclacitinib. Of these 283 dogs, two dogs were withdrawn from study due to suspected treatment-related adverse reactions: one dog that had an intense flare-up of dermatitis and severe secondary pyoderma after 19 days of oclacitinib administration, and one dog that developed generalized demodicosis after 28 days of oclacitinib administration. Two other dogs on oclacitinib were withdrawn from study due to suspected or confirmed malignant neoplasia and subsequently euthanized, including one dog that developed signs associated with a heart base mass after 21 days of oclacitinib administration, and one dog that developed a Grade III mast cell tumor after 60 days of oclacitinib administration.
One of the 147 dogs in the placebo group developed a Grade I mast cell tumor and was withdrawn from the masked study. Additional dogs receiving oclacitinib were hospitalized for diagnosis and treatment of pneumonia (one dog), transient bloody vomiting and stool (one dog), and cystitis with urolithiasis (one dog).
In the 283 dogs that received oclacitinib, the following additional clinical signs were reported after beginning of the treatment (percentage of dogs with at least one report of the clinical sign as a non-pre-existing finding): pyoderma (12.0%), non-specified dermal lumps (12.0%), otitis (9.9%), vomiting (9.2%), diarrhea (6.0%), histiocytoma (3.9%), cystitis (3.5%), anorexia (3.2%), lethargy (2.8%), yeast skin infections (2.5%), pododermatitis (2.5%), lipoma (2.1%), polydipsia (1.4%), lymphadenopathy (1.1%), nausea (1.1%), increased appetite (1.1%), aggression (1.1%), and weight loss (0.7%).
Continuation Field Study
After completing oclacitinib field studies, 239 dogs enrolled in an unmasked (no placebo control), continuation therapy study receiving oclacitinib tablets for an unrestricted period of time. Mean time on this study was 372 days (range 1 to 610 days).
Of these 239 dogs, one dog developed demodicosis following 273 days of oclacitinib administration. One dog developed dermal pigmented viral plaques following 266 days of oclacitinib administration. One dog developed a moderately severe bronchopneumonia after 272 days of oclacitinib administration; this infection resolved with antimicrobial treatment and temporary discontinuation of oclacitinib. One dog was euthanized after developing abdominal ascites and pleural effusion of unknown etiology after 450 days of oclacitinib administration. Six dogs were euthanized because of suspected malignant neoplasms: including thoracic metastatic, abdominal metastatic, splenic, frontal sinus, and intracranial neoplasms, and transitional cell carcinoma after 17, 120, 175, 49, 141, and 286 days of oclacitinib administration, respectively. Two dogs each developed a Grade II mast cell tumor after 52 and 91 days of oclacitinib administration, respectively. One dog developed low-grade B-cell lymphoma after 392 days of oclacitinib administration. Two dogs each developed an apocrine gland adenocarcinoma (one dermal, one anal sac) after approximately 210 and 320 days of oclacitinib administration, respectively. One dog developed a low-grade oral spindle cell sarcoma after 320 days of oclacitinib administration.
Clinical Pharmacology
Mechanism of Action
Oclacitinib inhibits the function of a variety of pruritogenic cytokines and pro-inflammatory cytokines, as well as cytokines involved in allergy that are dependent on JAK1 or JAK3 enzyme activity, whereas it has little effect on cytokines involved in hematopoiesis that are dependent on JAK2. Oclacitinib is not a corticosteroid or an antihistamine.
Pharmacokinetics
In a bioequivalence study, APOQUEL chewable tablets were administered orally in dogs at a dose ranging from 0.54 to 0.9 mg oclacitinib/kg body weight. The observed mean Cmax was 352 ng/mL (ranging from 207 to 860 ng/mL), which occurred approximately 1.7 hours (tmax) post dosing. The Cmax values were slightly lower after administration of APOQUEL chewable tablets than in the same dogs receiving the APOQUEL original tablets (mean 475 ng/mL). Maximum plasma concentrations (tmax) occurred later with the chewable tablet than the original tablet (mean 1.7 vs 1.2 h, respectively). Overall exposure was similar between the groups, with mean AUC0-t(last) of 3260 and 3000 ng•h/mL for APOQUEL original tablets and APOQUEL chewable tablets, respectively. The mean half-life (t1/2) values were also similar across groups at 4.35 and 4.81 hours for APOQUEL original tablets and APOQUEL chewable tablets, respectively.
From previous pharmacokinetic studies with oclacitinib, the half-life (t1/2) was 4.8 hours in plasma, the total apparent body oclacitinib clearance from plasma was low - 316 mL/h/kg bodyweight (5.3 mL/min/kg bodyweight), and the apparent volume of distribution at steady-state was 942 mL/kg bodyweight. Oclacitinib exhibits low protein binding with 66.3% to 69.7% bound in fortified canine plasma at nominal concentrations ranging from 10 to 1,000 ng/mL.
Oclacitinib is metabolized in the dog to multiple metabolites. One major oxidative metabolite was identified in plasma and urine. Overall, the major clearance route is metabolism, with minor contributions from renal and biliary elimination. Inhibition of canine cytochrome P450 enzymes by oclacitinib is minimal; the inhibitory concentrations (IC50s) are 50-fold greater than the observed Cmax values at the use dose. Therefore, the risk of metabolic drug-drug interactions due to oclacitinib inhibition is very low.
Efficacy:
In an interleukin (IL)-31 challenge study, the efficacy of APOQUEL chewable tablets was compared to a negative control and APOQUEL original tablets. On Day 0, 7 dogs in each group received 0.4-0.6 mg oclacitinib/kg bodyweight or a placebo. Approximately 45 minutes after dosing, each dog was administered IL-31 (2.5 µg/kg) to induce pruritus. Dogs were observed 1 hour after dosing for pruritic behaviour during a 120-minute period, and a pruritus score (0 or 1) was assigned. Both APOQUEL original tablets and APOQUEL chewable tablets showed a significantly different total pruritus score compared with placebo (p = 0.0069 and 0.0113, respectively). The efficacy of the two APOQUEL dosage forms was comparable over the period of the evaluation.
Palatability
The palatability of APOQUEL chewable tablets was evaluated in dogs in a US multi-location field trial. One hundred twenty-one (121) client-owned dogs were dosed with APOQUEL chewable tablets at a dose of 0.4-0.6 mg/kg twice daily for 7 days and evaluated for voluntary acceptance/intake of the product. Over the course of the study, a total of 1673 doses were administered. Dogs freely consumed 1533 doses (91.6%) within 5 minutes of offering from an empty bowl or owner’s hand. Of the 8.4% of doses unconsumed within 5 minutes, 8.0% of doses were administered with a treat/food or forced intake and 0.4% of doses were not consumed at all.
Control of Pruritus Associated with Allergic Dermatitis
A double-masked, 30-day, controlled study was conducted in the U.S. at 26 veterinary hospitals. The study enrolled 436 client-owned dogs with a history of allergic dermatitis attributed to one or more of the following conditions: atopic dermatitis, flea allergy, food allergy, contact allergy, and other/unspecified allergic dermatitis. Dogs were randomized to treatment with oclacitinib (216 dogs: tablets administered at a dose of 0.4-0.6 mg/kg twice daily) or placebo (220 dogs: vehicle control, tablets administered twice daily). During the study, dogs could not be treated with other drugs that could affect the assessment of pruritus or dermal inflammation such as corticosteroids, anti-histamines, or cyclosporine. Treatment success for each dog was defined as at least a 2 cm decrease from baseline on a 10 cm visual analog scale (VAS) in pruritus, assessed by the Owner, on at least 5 of the 7 evaluation days. The estimated proportion of dogs with Treatment Success was greater and significantly different for the oclacitinib-group compared to the placebo group.
Owner-Assessed Pruritus VAS Treatment Success, Allergic Dermatitis
|
Efficacy Parameter |
Oclacitinib (n = 203) |
Placebo (n = 204) |
P-value |
|
Estimated Proportion of Dogs with Treatment Success |
0.67 |
0.29 |
p<0.0001 |
After one week of treatment, 86.4% of dogs in the oclacitinib group compared with 42.5% of placebo-group dogs had achieved a 2 cm reduction on the 10 cm Owner-assessed pruritus VAS. On each of the 7 days, mean Owner-assessed pruritus VAS scores were lower in dogs in the oclacitinib group (See Figure 1). Veterinarians used a 10 cm VAS scale to assess each dog’s dermatitis. After one week of treatment, the mean Veterinarian-assessed VAS dermatitis score for the dogs in the oclacitinib group was lower at 2.2 cm (improved from a baseline value of 6.2 cm) compared with the placebo group mean score of 4.9 cm (from a baseline value of 6.2 cm). For dogs that continued oclacitinib treatment beyond one week, the Veterinarian-assessed dermatitis scores continued to improve through study end at Day 30.
Figure 1: Owner Assessed Pruritus VAS Scores by treatment for Days 0-7

Control of Atopic Dermatitis
A double-masked, 112-day, controlled study was conducted in the U.S. at 18 veterinary hospitals. The study enrolled 299 client-owned dogs with atopic dermatitis. Dogs were randomized to treatment with oclacitinib (152 dogs: tablets administered at a dose of 0.4-0.6 mg/kg per dose twice daily for 14 days and then once daily) or placebo (147 dogs: vehicle control, tablets administered on the same schedule). During the study, dogs could not be treated with other drugs that could affect the assessment of effectiveness, such as corticosteroids, anti-histamines, or cyclosporine. Treatment success for pruritus for each dog was defined as at least a 2 cm decrease from baseline on a 10 cm visual analog scale (VAS) in pruritus, assessed by the Owner, on Day 28. Treatment success for skin lesions was defined as a 50% decrease from the baseline Canine Atopic Dermatitis Extent and Severity Index (CADESI) score, assessed by the Veterinarian, on Day 28. The estimated proportion of dogs with Treatment Success in Owner-assessed pruritus VAS score and in Veterinarian-assessed CADESI score was greater and significantly different for the APOQUEL group compared to the placebo group.
Estimated Proportion of Dogs with Treatment Success, Atopic Dermatitis
|
Efficacy Parameter |
Oclacitinib |
Placebo |
P-value |
|
Owner Assessed Pruritus VAS |
0.66 (n = 131) |
0.04 (n = 133) |
p<0.0001 |
|
Veterinarian Assessed CADESI |
0.49 (n = 134) |
0.04 (n = 134) |
p<0.0001 |
Compared to the placebo group, mean Owner-assessed pruritus VAS scores (on Days 1, 2, 7, 14, and 28) and Veterinarian-assessed CADESI scores (on Days 14 and 28) were lower (improved) in dogs in the oclacitinib group. By Day 30, 86% (127/147) of the placebo group dogs and 15% (23/152) of the dogs in the oclacitinib group withdrew from the masked study because of worsening clinical signs and had the option to enroll in an unmasked study and receive oclacitinib. For dogs that continued oclacitinib treatment beyond one month, the mean Owner-assessed pruritus VAS scores and Veterinarian-assessed CADESI scores continued to improve through study end at Day 112.
Animal Safety:
Margin of Safety in 12-Month Old Dogs
Oclacitinib was administered to healthy, one-year-old Beagle dogs twice daily for 6 weeks, followed by once daily for 20 weeks, at 0.6 mg/kg (1X maximum exposure dose, 8 dogs), 1.8 mg/kg (3X, 8 dogs), and 3.0 mg/kg (5X, 8 dogs) for 26 weeks. Eight dogs received placebo (empty gelatin capsule) at the same dosage schedule. Clinical observations that were considered likely to be related to oclacitinib-included papillomas and a dose-dependent increase in the number and frequency of interdigital furunculosis (cysts) on one or more feet during the study. Additional clinical observations were primarily related to the interdigital furunculosis and included dermatitis (local alopecia, erythema, abrasions, scabbing/crusts, and edema of feet) and lymphadenopathy of peripheral nodes. Microscopic findings considered to be oclacitinib-related included decreased cellularity (lymphoid) in Gut-Associated Lymphoid Tissue (GALT), spleen, thymus, cervical and mesenteric lymph node; and decreased cellularity of sternal and femoral bone marrow. Lymphoid hyperplasia and chronic active inflammation were seen in lymph nodes draining feet affected with interdigital furunculosis. Five oclacitinib-treated dogs had microscopic evidence of mild interstitial pneumonia.
Clinical pathology findings considered to be oclacitinib-related included mild, dose-dependent reduction in hemoglobin, hematocrit, and reticulocyte counts during the twice daily dosing period with decreases in the leukocyte subsets of lymphocytes, eosinophils, and basophils. Total proteins were decreased over time primarily due to the albumin fraction.
Vaccine Response Study
An adequate immune response (serology) to killed rabies (RV), modified live canine distemper virus (CDV), and modified live canine parvovirus (CPV) vaccination was achieved in eight 16-week old vaccine naïve puppies that were administered oclacitinib at 1.8 mg/kg (3X maximum exposure dose) twice daily for 84 days. For modified live canine parainfluenza virus (CPI), < 80% (6 of 8) of the dogs achieved adequate serologic response. Clinical observations that were considered likely to be related to oclacitinib treatment included enlarged lymph nodes, interdigital furunculosis, cysts, and pododermatitis. One oclacitinib-treated dog (26-weeks-old) was euthanized on Day 74 after physical examination revealed the dog to be febrile, lethargic, with pale mucous membranes and frank blood in stool. Necropsy revealed pneumonia of short duration and evidence of chronic lymphadenitis of mesenteric lymph nodes. During the three-month recovery phase to this study, one oclacitinib-treated dog (32-weeks old) was euthanized on Day 28 due to clinical signs which included enlarged prescapular lymph nodes, bilateral epiphora, lethargy, mild dyspnea, and fever. The dog showed an elevated white blood cell (WBC) count. Necropsy revealed lesions consistent with sepsis secondary to immunosuppression. Bone marrow hyperplasia was consistent with response to sepsis.
Margin of Safety in 6 Month Old Dogs
A margin of safety study in 6-month-old dogs was discontinued after four months due to the development of bacterial pneumonia and generalized demodex mange infections in dogs in the high dose (3X and 5X) treatment groups, dosed at 1.8 and 3.0 mg/kg oclacitinib twice daily, for the entire study.
Storage
APOQUEL chewable tablets should be stored at controlled room temperature between 15 to 25°C, with excursions permitted up 40°C.
Presentation: APOQUEL chewable tablets contain 3.6 mg, 5.4 mg, or 16 mg of oclacitinib (as oclacitinib maleate) per chewable tablet. Each strength is packaged in 100 count bottles. Each chewable tablet is pentagon shaped, scored on both sides and have a dose descriptor (S S, M M or L L) debossed on one face across the score line. The S (small), M (medium) and L (large) markings correspond to the strengths of 3.6 mg, 5.4 mg and 16 mg respectively. The chewable tablets can be divided into equal halves.
Zoetis® is a registered trademark and Apoquel is a trademark of Zoetis or its licensors.
Zoetis Canada Inc., Kirkland QC H9H 4M7
10024853-11-0
40041424
July 2023
CPN: 1198589.0
16,740 TRANS-CANADA HIGHWAY, KIRKLAND, QC, H9H 4M7
| Order Desk: | 800-663-8888 | |
| Technical Services Canada: | 800-461-0917 | |
| Technical Services USA: | 800-366-5288 | |
| Website: | www.zoetis.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
