Epinephrine Nasal Solution Prescribing Information
Package insert / product label
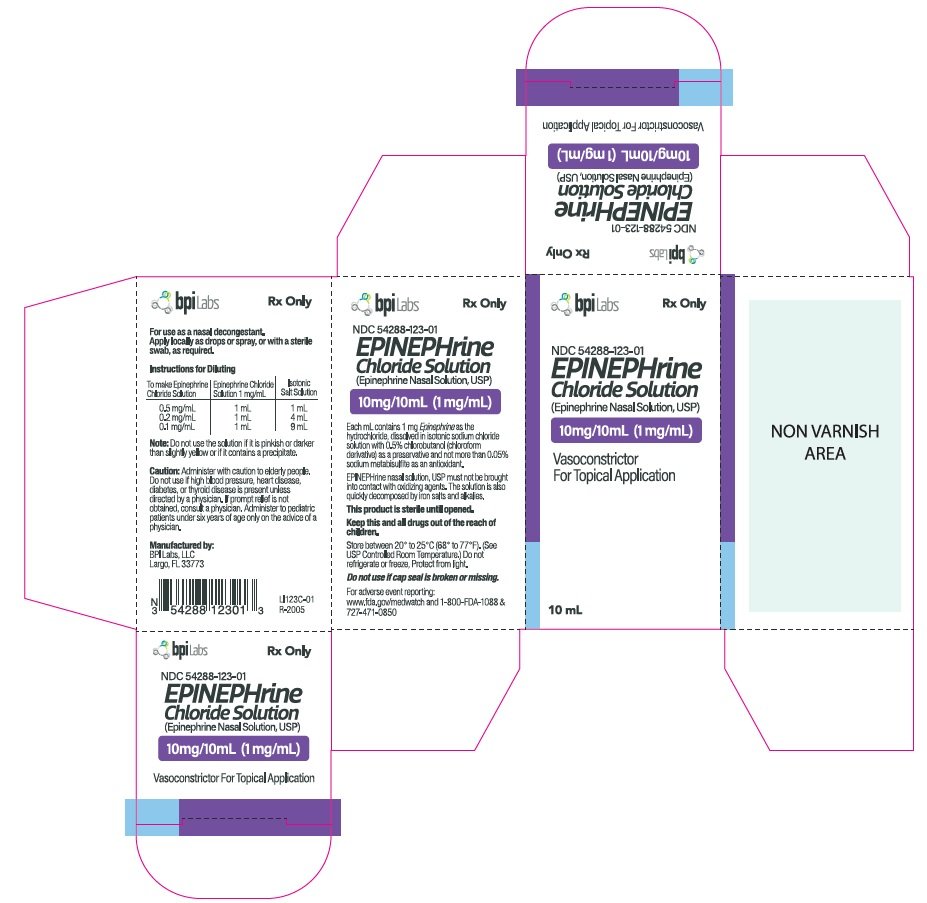
Generic name: epinephrine hydrochloride
Dosage form: nasal solution
On This Page
Warnings and Precautions
Caution: Administer with caution to elderly people. Do not use if high blood pressure, heart disease, diabetes, or thyroid disease is present unless directed by a physician. If prompt relief is not obtained, consult a physician. Administer to pediatric patients under six years of age only on the advice of a physician.
Indications and Usage for Epinephrine Nasal Solution
For use as a nasal decongestant.Apply locally as drops or spray, or with a sterile swab, as required.
Instructions for diluting
| To make Epinephrine Chloride Solution | Epinephrine Chloride Solution 1 mg/mL | Isotonic Salt Solution |
| 0.5 mg/mL | 1 mL | 1 mL |
| 0.2 mg/mL | 1 mL | 4 mL |
| 0.1 mg/mL | 1 mL | 9 mL |
Epinephrine Nasal Solution Dosage and Administration
Each mL contains 1 mg Epinephrine as the hydrochloride, dissolved in isotonic sodium chloride solution with 0.5% chlorobutanol (chloroform derivative) as a preservative and not more than 0.05% sodium metabisulfite as an antioxidant.EPINEPHrine nasal solution, USP must not be brought into contact with oxidizing agents.The solution is also quickly decomposed by iron salts and alkalies.
Adverse Reactions/Side Effects
To report SUSPECTED ADVERSE REACTIONS, contact BPI Labs, LLC at 1-727-471-0850 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
How is Epinephrine Nasal Solution supplied
EPINEPhrine Chloride Solution 10mg/10ml (1mg/1ml) in 10ml vial.NDC 54288-123-01
This product is sterile until opened.
Keep this and all drugs out of the reach of children.
Store between 20° to 25°C (68° to 77°F). (See USP Controlled Room Temperature.) Do not refrigerate or freeze, Protect from light.
Do not use if cap seal is broken or missing.
Manufactured by:
BPI Labs, LLC
Largo, FL 33773.
Rev: 08/2020
LI123C-01
R-2005
| EPINEPHRINE CHLORIDE SOLUTION
epinephrine nasal solution solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - BPI Labs, LLC (078627620) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BPI Labs, LLC | 078627620 | manufacture(54288-123) , label(54288-123) | |