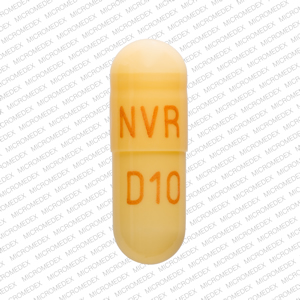
NVR D10 Pill - brown capsule/oblong, 18mm
Pill with imprint NVR D10 is Brown, Capsule/Oblong and has been identified as Dexmethylphenidate Hydrochloride Extended-Release 10 mg. It is supplied by Sandoz Inc.
Dexmethylphenidate is used in the treatment of ADHD and belongs to the drug class CNS stimulants. Risk cannot be ruled out during pregnancy. Dexmethylphenidate 10 mg is classified as a Schedule 2 controlled substance under the Controlled Substance Act (CSA).
Images for NVR D10
Dexmethylphenidate Hydrochloride Extended-Release
- Imprint
- NVR D10
- Strength
- 10 mg
- Color
- Brown
- Size
- 18.00 mm
- Shape
- Capsule/Oblong
- Availability
- Prescription only
- Drug Class
- CNS stimulants
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- 2 - High potential for abuse
- Labeler / Supplier
- Sandoz Inc.
- Inactive Ingredients
-
ammonio methacrylate copolymer type A,
ferric oxide yellow,
gelatin,
methacrylic acid - methyl methacrylate copolymer (1:1),
polyethylene glycol,
magnesium silicate,
titanium dioxide,
triethyl citrate
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 00781-2683 | Sandoz Inc. |
| 00078-0431 | Novartis Pharmaceuticals Corporation |
| 54868-5682 (Discontinued) | Physicians Total Care Inc. (repackager) |
| 35356-0159 | Lake Erie Medical and Surgical Supply (repackager) |
Related images for "NVR D10"
More about dexmethylphenidate
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (292)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Drug class: CNS stimulants
- Breastfeeding
- En español
Patient resources
- Dexmethylphenidate drug information
- Dexmethylphenidate (Advanced Reading)
- Dexmethylphenidate Tablets
- Dexmethylphenidate Extended-Release Capsules
Other brands
Professional resources
- Dexmethylphenidate/Serdexmethylphenidate monograph
- Dexmethylphenidate (FDA)
- Dexmethylphenidate ER Capsule (FDA)
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.