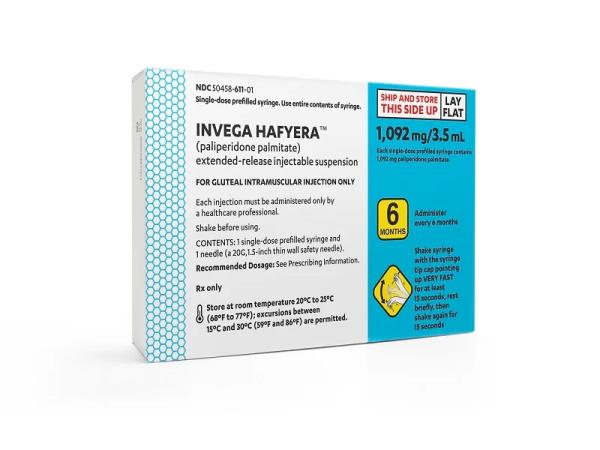
Invega Hafyera Dosage
Generic name: paliperidone palmitate 1092mg in 3.5mL
Dosage form: injection, suspension, extended release
Drug class: Atypical antipsychotics
Medically reviewed by Drugs.com. Last updated on Oct 17, 2023.
Important Dosage and Administration Information
- INVEGA HAFYERA must be administered as a gluteal intramuscular injection by a healthcare professional once every 6 months. Do not administer by any other route [see Dosage and Administration (2.4, 2.5)].
- Initiate INVEGA HAFYERA only after adequate treatment has been established with either:
- A once-a-month paliperidone palmitate extended-release injectable suspension (e.g., INVEGA SUSTENNA), referred to as PP1M, once monthly for at least four months; or
- An every-three-month paliperidone palmitate extended-release injectable suspension (e.g., INVEGA TRINZA), referred to as PP3M, once every three months for at least one three-month injection cycle.
- See Prescribing Information of the PP1M and PP3M products for the recommended dosage of these products.
Recommended Dosage for INVEGA HAFYERA
Switching to INVEGA HAFYERA from a PP1M Product
The recommended initial INVEGA HAFYERA dose is based on the previous PP1M dose (see Table 1). Initiate INVEGA HAFYERA when the next PP1M dose is scheduled. INVEGA HAFYERA may be administered up to 1 week before or 1 week after the next scheduled PP1M dose. When switching from PP1M to INVEGA HAFYERA, the two injection cycles immediately preceding the switch should be the same dosage strength before starting INVEGA HAFYERA.
| Last Dose of PP1M† | Initial Dose of INVEGA HAFYERA |
|---|---|
|
|
| 156 mg | 1,092 mg |
| 234 mg | 1,560 mg |
Switching to INVEGA HAFYERA from a PP3M Product
The recommended initial INVEGA HAFYERA dose is based on the previous PP3M dose (see Table 2). Initiate INVEGA HAFYERA when the next PP3M dose is scheduled. INVEGA HAFYERA may be administered up to 2 weeks before or 2 weeks after the next scheduled PP3M dose.
| Last Dose of PP3M† | Initial Dose of INVEGA HAFYERA |
|---|---|
|
|
| 546 mg | 1,092 mg |
| 819 mg | 1,560 mg |
Dosing Interval and Dosage Adjustments of INVEGA HAFYERA
Following the initial dose, administer INVEGA HAFYERA once every 6 months.
If needed, dosage adjustment can be made every 6 months between the dose of 1,092 mg to 1,560 mg based on individual response and tolerability. Because of the potential longer duration of INVEGA HAFYERA, the patient's response to an adjusted dose may not be apparent for several months [see Clinical Pharmacology (12.3)].
Missed Doses
Dosing Window
To avoid a missed dose, patients may be given the injection up to 2 weeks before or 3 weeks after the scheduled 6-month dose.
Missed Dose
If a dose of INVEGA HAFYERA is missed, re-initiate with a PP1M product using the re-initiation regimens described in Tables 3 and 4.
More than 6 Months and 3 Weeks, up to but Less than 8 Months Since Last Dose
If more than 6 months and 3 weeks but less than 8 months have elapsed since the last dose of INVEGA HAFYERA, do not administer the next dose of INVEGA HAFYERA. Instead, use the re-initiation regimen shown in Table 3:
| Last Dose of INVEGA HAFYERA | Administer PP1M Product* into deltoid muscle | Administer INVEGA HAFYERA into gluteal muscle |
|---|---|---|
| Day 1 | 1 month after Day 1 | |
|
||
| 1,092 mg | 156 mg | 1,092 mg |
| 1,560 mg | 234 mg | 1,560 mg |
8 Months Up to and including 11 Months Since Last Dose
If 8 months but up to and including 11 months have elapsed since the last dose of INVEGA HAFYERA, do not administer the next dose of INVEGA HAFYERA. Instead, use the re-initiation regimen shown in Table 4:
| Last dose of INVEGA HAFYERA | Administer PP1M Product* into deltoid muscle | Administer INVEGA HAFYERA into gluteal muscle | |
|---|---|---|---|
| Day 1 | Day 8 | 1 month after Day 8 | |
|
|||
| 1,092 mg | 156 mg | 156 mg | 1,092 mg |
| 1,560 mg | 156 mg | 156 mg | 1,560 mg |
More than 11 Months Since Last Dose
If more than 11 months have elapsed since the last dose of INVEGA HAFYERA, re-initiate treatment with a PP1M product as described in the prescribing information for that product. INVEGA HAFYERA can then be resumed after the patient has been adequately treated with a PP1M product for at least 4 months.
Instructions for Preparation and Administration
- To be prepared and administered by a healthcare provider only.
- Read the instructions for preparation and administration below and consider referring to the separate Healthcare Provider "Instructions for Use" for preparation and administration considerations.
- For gluteal intramuscular injection only. Do not inject by any other route. As a universal precaution, always wear gloves.
- Inspect INVEGA HAFYERA for particulate matter and discoloration prior to administration.
- Do not mix with any other product or diluent.
- After shaking, INVEGA HAFYERA should appear uniform, thick and milky white.
- Do not use needles from the PP1M or PP3M products or other commercially-available needles to reduce the risk of blockage.
- Avoid inadvertent injection into a blood vessel. Administer the dose in a single injection; do not administer the dose in divided injections. Inject slowly, deep into the upper-outer quadrant of the gluteal muscle. Future injections should be alternated between the two gluteal muscles.
Incomplete Administration
- Proper shaking can reduce the likelihood for an incomplete injection. Storing the carton in a horizontal orientation improves the ability to resuspend this highly concentrated product [see How Supplied/Storage and Handling (16)].
- Follow the full instructions for preparation and administration to avoid an incomplete injection.
- In the event of an incompletely administered dose, do not re-inject the dose remaining in the syringe and do not administer another dose of INVEGA HAFYERA.
- Closely monitor and treat the patient with oral paliperidone supplementation as clinically appropriate until the next scheduled 6-month injection of INVEGA HAFYERA. See Prescribing Information of the oral paliperidone product for the recommended dosage of these products.
|
Administer every 6 months |
INVEGA HAFYERA™ (paliperidone palmitate) Shake syringe with the syringe tip cap pointing up VERY FAST for at least 15 seconds, rest briefly, then shake again for 15 seconds. For Gluteal Intramuscular injection only. |
 |
|
| Preparation | INVEGA HAFYERA requires longer and faster shaking than once-a-month paliperidone palmitate extended-release injectable suspension (e.g., INVEGA SUSTENNA). INVEGA HAFYERA must be administered by a healthcare professional as a single injection. Do not divide dose into multiple injections. INVEGA HAFYERA is intended for gluteal intramuscular use only. Inject slowly, deep into the muscle taking care to avoid injection into a blood vessel. |
| Dosing | Administer INVEGA HAFYERA once every 6 months. |
| Thin Wall Safety Needle | Thin wall safety needle is designed to be used with INVEGA HAFYERA. Therefore, it is important to only use the needle provided in the INVEGA HAFYERA suspension kit. |
| Dose pack contents Prefilled Syringe |
|
|
Thin Wall Safety Needle |
|
| 1. Prepare for the injection: this highly concentrated product requires specific steps to resuspend | |
| Hold syringe with the tip cap pointing up |
Syringe tip cap pointing up |
|
Shake syringe VERY FAST for at least 15 seconds, rest briefly, then shake again for 15 seconds
|
|
| Proceed to the next step immediately after shaking. | |
Check suspension for solid product |
|
Mixed well |
Not mixed well |
|
|
| Shake syringe with the syringe tip cap pointing up VERY FAST for at least 15 seconds, rest, then shake again for 15 seconds. | |
| Open needle pouch | Peel off the pouch cover. Place pouch with the needle inside on a clean surface. |
 |
|
| Remove syringe tip cap and attach needle | Hold the syringe with the tip cap pointing up. Twist and pull off the cap. Hold the syringe by the luer connection. Twist it into the safety needle with a gentle clockwise twisting motion. |  |
 |
| Pull back plunger Hold the syringe upright. Gently pull back the plunger to clear the syringe tip of any solid product. This will make pressing the plunger easier during the injection. |
 |
| Remove air bubbles Press the plunger carefully until a drop of liquid comes out of the needle tip. |
 |
| 2. Slowly inject entire content and confirm | |
| Select and clean a gluteal injection site Wipe the gluteal site with an alcohol swab and allow it to dry. Do not touch, fan or blow on the injection site after you have cleaned it. |
 |
| Remove needle sheath Pull the needle sheath away from the needle in a straight motion. Do not twist the sheath, as this may loosen the needle from the syringe. |
 |
| Slowly inject and confirm Use slow, firm, consistent pressure to press the plunger completely. This should take approximately 30 seconds. Continue to press the plunger if you feel resistance. This is normal. |
|
|
|
 |
| Remove needle from the muscle. | |
| 3. After the injection | |
| Secure needle After the injection is complete, use your thumb or a flat surface to secure the needle in the safety device. The needle is secure when you hear a "click" sound. |
 |
| Dispose of properly and check injection site Dispose of the syringe in an approved sharps container. There may be a small amount of blood or liquid at the injection site. Hold pressure over the skin with a cotton ball or gauze pad until any bleeding stops. Do not rub the injection site. If needed, cover injection site with a bandage. |
 |
Frequently asked questions
- Invega Sustenna vs Invega Trinza vs Invega Hafyera. What's the difference?
- Can you drink alcohol while taking Invega Sustenna?
- How to transition to Invega Trinza from Invega Sustenna ?
- How do you give an Invega Sustenna injection?
- How to transition to Invega Hayfera from Invega Trinza or Invega Sustenna?
- How is it administered?
More about Invega Hafyera (paliperidone)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Drug images
- Side effects
- During pregnancy
- FDA approval history
- Drug class: atypical antipsychotics
- Breastfeeding
- En español
Patient resources
Other brands
Invega, Invega Sustenna, Invega Trinza
Professional resources
Other brands
Invega, Invega Sustenna, Invega Trinza
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.