Cervidil Dosage
Generic name: DINOPROSTONE 10mg in 241mg
Dosage form: vaginal insert
Drug class: Uterotonic agents
Medically reviewed by Drugs.com. Last updated on Jun 2, 2023.
Dosage Instructions
Administer one CERVIDIL insert (10 mg) intravaginally for use up to 12 hours (approximately 0.3 mg of dinoprostone is released per hour) [see Dosage and Administration (2.2)].
Monitor uterine activity, fetal status, and the progression of cervical dilatation and effacement with the use of CERVIDIL. Remove CERVIDIL 12 hours after insertion with the onset of active labor, prior to an amniotomy, occurrence of uterine tachysystole, uterine hypersystole/hypertonicity, or fetal distress [see Warnings and Precautions (5.4)]. Remove CERVIDIL at least 30 minutes prior to administering an oxytocic agent [see Warnings and Precautions (5.4) and Drug Interactions (7)].
Preparation and Administration Instructions
CERVIDIL should be administered only by trained obstetrical personnel in a hospital setting with appropriate obstetrical care facilities.
Preparation and Administration Instructions
- Keep CERVIDIL frozen until ready for use. Do not warm CERVIDIL prior to vaginal insertion.
- Tear open the individually-wrapped foil package containing CERVIDIL along the tear mark. Never open the package using scissors or other sharp objects because this may damage the knitted polyester retrieval system. Do not cut the retrieval system and do not use CERVIDIL unless its retrieval system is intact.
- Immediately after opening the package, insert CERVIDIL transversely, in the posterior fornix of the vagina (see Figure 1). If necessary, use a minimal amount of water-miscible lubricant to assist vaginal insertion. Do not permit excess contact or coating with the lubricant, as this could prevent release of dinoprostone from the vaginal insert. Insertion does not require sterile conditions.
Figure 1: Placement of CERVIDIL in the Posterior Vaginal Fornix 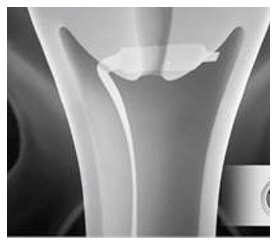
- Tuck some of the excess retrieval system into the vagina to avoid movement of CERVIDIL away from the proper position; however, leave a small amount of the retrieval system outside the vagina to aid in retrieval.
- Instruct women to remain in a recumbent position during insertion of CERVIDIL and for 2 hours afterward. Women may be ambulatory 2 hours after insertion; however, ensure that the insert remains in place.
More about Cervidil (dinoprostone topical)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (18)
- Side effects
- During pregnancy
- Drug class: uterotonic agents
- Breastfeeding
- En español
Patient resources
Other brands
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.

