Aralast NP Dosage
Generic name: .ALPHA.1-PROTEINASE INHIBITOR HUMAN 16mg in 1mL;
Dosage form: injection
Drug class: Miscellaneous respiratory agents
Medically reviewed by Drugs.com. Last updated on May 15, 2023.
For Intravenous Use Only
Dosage
- Dose ranging studies using efficacy endpoints have not been performed.
- Administer 60 mg/kg body weight of ARALAST NP once weekly by intravenous infusion.
Reconstitution
- Use aseptic technique.
- Allow ARALAST NP and diluent to reach room temperature before reconstitution.
- Remove caps from the diluent and product vials.
- Swab the exposed stopper surfaces with alcohol.
- Remove cover from one end of the double-ended transfer needle. Insert the exposed end of the needle through the center of the stopper in the diluent vial.
- Remove plastic cap from the other end of the double-ended transfer needle now seated in the stopper of the diluent vial. To reduce any foaming, invert the vial of diluent and insert the exposed end of the needle through the center of the stopper in the product vial at an angle, making certain that the diluent vial is always above the product vial. The angle of insertion directs the flow of diluent against the side of the product vial. Refer to Figure 1 below. The vacuum in the vial is sufficient to allow transfer of all of the diluent.
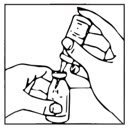
Figure 1
- Disconnect the two vials by removing the diluent vial from the transfer needle. This allows any remaining low pressure in the product vial to equalize. Next, remove the double-ended transfer needle from the product vial and discard the needle into the appropriate safety container.
- Let the vial stand until most of the contents is in solution, then GENTLY swirl until the powder is completely dissolved. Reconstitution requires no more than five minutes for a 0.5 gram vial and no more than 10 minutes for a 1 gram vial.
Note: Do not shake the content of the vial. Do not invert the vial until ready to withdraw content. - Reconstituted product is a colorless or slightly yellow to yellow-green solution.
- A few small visible particles may occasionally remain in the reconstituted product. These will be removed by the sterile 20 micron filter needle supplied with the product.
- Place the product vial on a stable flat surface.
- Open the blister pack of the 20 micron filter needle.
- Twist to remove the protective cap from the Luer adapter of the filter needle.
- Attach a single-use syringe (not provided) to the Luer adapter of the filter needle.
- Remove the protective cap from the filter needle and draw back plunger of the syringe to admit air into syringe. Insert the filter needle through the center of the stopper in product vial.
- Push the plunger to admit air in the product vial.
- Invert the system (product vial with syringe attached) so that product vial is on top.
- Withdraw the reconstituted product into the syringe.
- Remove the syringe from the filter needle and product vial. Remove filter needle from product vial and discard the needle into the appropriate sharps container.
Administration
For intravenous infusion
- Product from multiple vials may be pooled to achieve the calculated dose [See Dosage (2.1)].
- Several vials may be pooled using a separate needle (not provided) into an empty, sterile intravenous solution container using aseptic technique.
- Inspect the filtered reconstituted product visually for particulate matter and discoloration prior to administration. Administer ARALAST NP within three hours after reconstitution to reduce the risk of harmful microbial growth. Discard any unused contents.
- Administer ARALAST NP alone, without mixing with other agents or diluting solutions.
Infusion Rate
- Administer ARALAST NP at a rate not to exceed 0.2 mL per kg body weight per minute, and as determined by the response and comfort of the patient.
- Reduce the infusion rate or halt the infusion if adverse reactions occur. Resume the infusion at a rate tolerated by the patient after symptoms subside.
More about Aralast NP (alpha 1-proteinase inhibitor)
- Compare alternatives
- Pricing & coupons
- Side effects
- During pregnancy
- Drug class: miscellaneous respiratory agents
- En español
Patient resources
Other brands
Prolastin-C, Prolastin, Glassia, Zemaira
Professional resources
Other brands
Other formulations
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.


