Albutein 25% Dosage
Generic name: Albumin Human 12.5g in 50mL
Dosage form: injection
Drug class: Plasma expanders
Medically reviewed by Drugs.com. Last updated on Jan 4, 2024.
For Intravenous Use Only
Dosage
Adjust the concentration, dosage and infusion rate of the albumin preparation to the patient's individual requirements
The dose required depends on the patient's body weight, severity of injury/illness and on continuing fluid and protein losses. Use adequacy of circulating blood volume, not plasma albumin levels, to determine the dose required.
| Indication | Dose |
| Hypovolemia | Adults: Initial dose of 25 g. If hemodynamic stability is not achieved within 15 to 30 minutes, an additional dose may be given. Hemodilution may follow administration of ALBUTEIN 25%. Anemia resulting from hemorrhage should be corrected by administration of compatible red blood cells or compatible whole blood. For acute liver failure: initial dose of 12 to 25 g. An infusion rate of 1-2 mL per minute is usually indicated. For renal dialysis, the initial dose should not exceed 25 g and patients should be carefully observed for signs of fluid overload. |
| Cardiopulmonary bypass procedures | Adults: Initial dose of 25 g. Additional amounts may be administered as clinically indicated. |
| Acute nephrosis | Adults: 25 g together with diuretic once a day for 7 - 10 days. |
| Hypoalbuminemia | Adults: 50 to 75 g For pre- and post-operative hypoproteinemia: 50 to 75 g. In burns, therapy usually starts with administration of large volumes of crystalloid solution to maintain plasma volume. After 24 hours: initial dose of 25 g and dose adjustment to maintain plasma protein concentration of 2.5 g per 100 mL or a serum protein concentration of 5.2 g per 100 mL. Third space protein loss due to infection: initial dose of 50 to 100 g. An infusion rate of 1-2 mL per minute is usually indicated in the absence of shock. Treatment should always be guided by hemodynamic response. |
| Ovarian hyperstimulation syndrome | Adults: 50 g to 100 g over 4 hours and repeated at 4-12 hour intervals as necessary, when infusion of normal saline fails to achieve or maintain hemodynamic stability and urine output. |
| Neonatal hyperbilirubinemia | 1 g per kilogram body weight prior to or during exchange transfusion. |
| Adult respiratory distress syndrome (ARDS) | Adults: 25 g over 30 minutes and repeated at 8 hours for 3 days, if necessary. |
| Prevention of central volume depletion after paracentesis due to cirrhotic ascites | Adults: 8 g for every 1000 mL of ascitic fluid removed. |
Administration
Intravenous use only
- Some moisture or condensation may be observed in the protective overwrap. This is normal and does not affect the quality or safety of the albumin solution.
- Check the inner bag for any leaks prior to use by squeezing it firmly. If leaks are detected, discard the solution.
- ALBUTEIN 25% is a clear and slightly viscous solution. Visually inspect for particulate matter and discoloration prior to administration. Do not use if the solution is turbid, if there is sediment in the container, or if the seal is broken.
- Warm product to room temperature before use if large volumes are administered.
- Do not add supplementary medication.
- Do not dilute with sterile water for injection. Acceptable diluents include 0.9% Sodium Chloride or 5% Dextrose in Water [see Warnings and Precautions (5.7)].
- ALBUTEIN 25% contains no preservatives. Once open, the product should be used within four hours. Discard unused portion.
- For single use. Any unused solution must be discarded.
CAUTION: Do not use bags in series connections. Such use could result in air embolism due to residual air being drawn from the primary bag before the administration of the fluid from the secondary bag is complete.
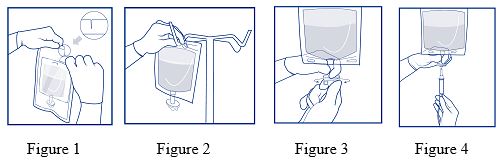
- After checking that protective overwrap is not damaged, remove it by tearing the slots at either end (refer to Figure 1).
- Suspend the inner bag from the eyelet support (refer to Figure 2).
- Holding the protective safety shield at the infusion port of the inner bag with one hand, use the free hand to exert light force to turn the twist-off opening about 90 degrees until it leaves the port (refer to Figure 3).
- Attach either a non-vented or vented administration set (refer to Figure 4). Adjust the infusion rate to the individual circumstances and the indication. Refer to the complete directions of administration set used.
More about Albutein (albumin human)
- Compare alternatives
- Pricing & coupons
- Side effects
- Dosage information
- During pregnancy
- Drug class: plasma expanders
- En español
Patient resources
Other brands
Alburx, Albuminar-25, Flexbumin, Albumarc, ... +12 more
Professional resources
- Albutein prescribing information
- Albutein 25% (FDA)
- Albutein 5% (FDA)
- Albumin Human (AHFS Monograph)
Other brands
Alburx, Albuminar-25, Flexbumin, Albuminex, ... +4 more
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.


