VetScan Canine Parvovirus Antigen Test Kit, Blot Test
This treatment applies to the following species:FOR THE RAPID QUALITATIVE DETECTION OF CANINE PARVOVIRUS ANTIGENS IN CANINE FECES
For Veterinary Use Only
Intended Use
The VetScan Canine Parvovirus Rapid Test is a visual and rapid test for the qualitative detection of canine parvovirus antigens in feces. This test is for veterinary in-vitro diagnostic use only.
Introduction
Canine parvovirus is a contagious virus that affects dogs mostly. The intestinal form of the disease causes severe vomiting and hemorrhagic diarrhea in puppies and young dogs (1,2). VetScan Canine Parvovirus Rapid Test employs a unique combination of monoclonal antibodies that give the test great specificity and sensitivity for the detection of parvovirus antigens.
MATERIALS AND REAGENTS PROVIDED
1. Test Device. Each sealed pouch contains a test strip in a plastic cassette and a desiccant.
2. Sample Dilution Device. Each consists of a sampling swab and 0.7mL of Extraction Buffer with sodium azide (0.02%) as a preservative.
3. Package Insert.
PRECAUTIONS AND WARNINGS
1. Parvovirus is infectious. Samples should be handled accordingly. The user should refer to the relevant section of the CDC-OHS manual “Biosafety in Microbiology and Biomedical Laboratories,” 5th Edition, 2007 for handling and disposal of the specimens, devices, and reagent.
2. Extraction buffer contains sodium azide, which may react with lead and copper plumbing to form explosive metal azides. Azide build-up may be avoided by flushing drains with large volumes of water after disposal.
3. The Test Device should remain in the sealed pouch until ready for use.
4. The Test Device and the Sample Dilution Device should be used when they are at room temperature (15°-27°C, or 59°-80°F)
5. Do not use kit or materials beyond expiration date.
6. Do not mix or interchange serials of VetScan Canine Parvovirus Rapid Test devices.
7. Use a new Test Device and a new Sample Dilution Device for each testing. Do not reuse devices.
SPECIMEN COLLECTION
1. Fresh samples should be used when possible. If samples cannot be used within 24 hours, they should be stored cold (2°-8°C or 36°-46°F) for up to two weeks, or kept frozen before being tested.
2. Some fecal samples contain mucous. In order to collect an accurate sample, avoid coating the swab completely with mucous material.
TEST PROCEDURE
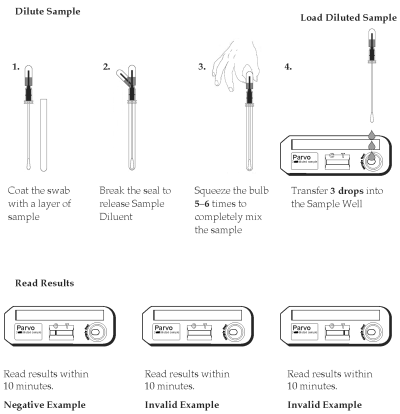
1. Remove the sampling swab from the dilution chamber of the Sample Dilution Device. Coat the sampling swab with a thin layer of fecal material or saturate the swab if the sample is liquid. Return the sampling swab back into the dilution chamber.
2. Break the seal of the Extraction Buffer storage bulb located on the top of the Sample Dilution Device by bending the bulb back and forth until the internal blue stick breaks at its base. Squeeze the bulb 5 - 6 times to thoroughly mix the sample with the Extraction Buffer.

3. Remove a Test Device from the protective pouch and place it on a flat surface.
4. Using the buffer storage bulb and the sampling swab as a pipette, transfer 3 drops (~150µL) of the diluted sample into the round sample well of the Test Device. Hold the pipette vertically to ensure proper sample volume. Start timing.
5. Read the results between 10 minutes and 15 minutes. Strong positive results may appear as soon as 1 minute, and weak positive results may take up to 10 minutes to appear. After 15 minutes, discard the Test Device because the results are no longer valid.
INTERPRETATION OF RESULTS
Negative Results
The result is negative when one red to pink line appears in the C (control) area.

Positive Results
The result is positive when two red to pink lines appear. One red/pink line will appear in the T (test) area and one in the C (control) area. A red to pink line with any intensity in the T (test) area should be considered positive. Colored lines may be lighter or darker than each other.
Invalid Results
The test is invalid when no colored line appears in the C (control) area, even if a red to pink line appears in the T (test) area. Repeat the test using a new cassette. Colored lines that appear after 15 minutes are not diagnostic and should be ignored.
Storage
The test kit is to be stored at room temperature (15°-27°C, or 59°-80°F) for the duration of the shelf life. Avoid the test kit being subjected to excessive heat or freezing for a long period of time.
Sensitivity And Specificity
In an USDA approved study involving 258 samples, the VetScan Canine Parvovirus Rapid Test correctly identified 93/96 hemagglutination inhibition (HI) positive canine fecal samples and 157/162 negative canine fecal samples. The discrepant results were resolved by PCR. The sensitivity and the specificity of the test was 96.9% and 96.9%, respectively.
PARVOVIRUS TEST PROCEDURE
REFERENCES
1. Lamm CG, Rezabek GB. Parvovirus infection in domestic companion animals. Vet Clin North Am Small Anim Pract. 2008 Jul;38(4):837-50, viii-ix.
2. Truyen U. Evolution of canine parvovirus--a need for new vaccines? Vet Microbiol. 2006 Oct 5;117(1):9-13.
For Technical Assistance Call: 800-822-2947
Manufacturer
SA Scientific, 4919 Golden Quail, San Antonio, TX 78240
U.S. Vet. License No. 373
Distributed by: Abaxis, Inc., 3240 Whipple Road, Union City, CA 94587
800-822-2947
www.abaxis.com
Authorized Representative In The European Community
ABAXIS Europe GmbH, Bunsenstr. 9-11, 64347 Griesheim, Germany
+49 6155 780 210
For patent information, see www.abaxis.com/about_us/patents
230-7002 Rev. D
Presentation: 10 and 20 test kits.
CPN: 1740002.1
Distributed by ZOETIS INC.
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-359-4414 | |
| Customer Service: | 888-963-8471 | |
| Website: | www.zoetis.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
