Vetmedin-CA1 (pimobendan) Chewable Tablets
This treatment applies to the following species: Company: Boehringer Ingelheim Animal Health
Company: Boehringer Ingelheim Animal Health
(pimobendan)
Chewable Tablets
Cardiac drug for oral use in dogs only
Vetmedin-CA1 (pimobendan) Chewable Tablets Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian. Use only as directed.
It is a violation of Federal law to use this product other than as directed in the labeling.
Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-556.
Description
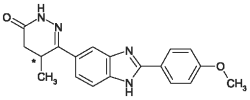
VETMEDIN-CA1 (pimobendan) is supplied as oblong half-scored chewable tablets containing 1.25 or 5 mg pimobendan per tablet. Pimobendan, a benzimidazole-pyridazinone derivative, is a non-sympathomimetic, non-glycoside inotropic drug with vasodilatative properties. Pimobendan exerts a stimulatory myocardial effect by a dual mechanism of action consisting of an increase in calcium sensitivity of cardiac myofilaments and inhibition of phosphodiesterase (Type III). Pimobendan exhibits vasodilating activity by inhibiting phosphodiesterase III activity. The chemical name of pimobendan is 4,5-dihydro-6-[2-(4-methoxyphenyl)-1H-benzimidazole-5-yl]-5-methyl-3(2H)-pyridazinone. The structural formula of pimobendan is:
Vetmedin-CA1 (pimobendan) Chewable Tablets Indications
VETMEDIN-CA1 (pimobendan) is indicated for the delay of onset of congestive heart failure in dogs with Stage B2 preclinical myxomatous mitral valve disease (2019 ACVIM Consensus Statement1).
Stage B2 preclinical myxomatous mitral valve disease (MMVD) refers to dogs with asymptomatic MMVD that have a moderate or loud mitral murmur due to mitral regurgitation and cardiomegaly.
Dosage and Administration
Always provide the Client Information Sheet to the dog owner with each prescription. VETMEDIN-CA1 should be administered orally at a total daily dose of 0.23 mg/lb (0.5 mg/kg) body weight, using a suitable combination of whole or half tablets. The total daily dose should be divided into 2 portions that are not necessarily equal, and the portions should be administered approximately 12 hours apart (i.e., morning and evening). The tablets are scored, and the calculated dosage should be provided to the nearest half tablet increment.Contraindications
Do not administer VETMEDIN-CA1 in cases of hypertrophic cardiomyopathy, aortic stenosis, or any other clinical condition where an augmentation of cardiac output is inappropriate for functional or anatomical reasons.
Do not administer VETMEDIN-CA1 to dogs with Stage A or B1 preclinical MMVD (2019 ACVIM Consensus Statement) due to the risk of cardiac pathology associated with exaggerated hemodynamic responses to VETMEDIN-CA1.
Warnings
User Safety Warnings: Not for use in humans. Keep this and all medications out of reach of children. Consult a physician in case of accidental ingestion by humans.
Animal Safety Warnings: Keep VETMEDIN-CA1 in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
At 3 and 5 times the recommended dosage, administered over a 6-month period of time, pimobendan caused an exaggerated hemodynamic response in the normal dog heart, which was associated with cardiac pathology (See Target Animal Safety).
Precautions
For use only in dogs with preclinical MMVD that have a moderate or loud mitral murmur due to mitral regurgitation and cardiomegaly (Stage B2 MMVD, 2019 ACVIM Consensus Statement). A diagnosis of MMVD should be made by means of a comprehensive physical and cardiac examination which should include radiography and echocardiography.Stage B2 cardiomegaly is diagnosed based on meeting all three of the following criteria:
● Radiographic vertebral heart score (VHS) >10.5, and
● Echocardiographic left atrium/aorta ratio (LA/Ao ratio) ≥1.6, and
● Echocardiographic left ventricular internal diastolic diameter normalized to body weight (LVIDDN) ≥1.7.
Echocardiographic examination is recommended in all cases to diagnose MMVD and confirm cardiomegaly. If therapy is initiated prior to the development of cardiomegaly, treated dogs are at risk for cardiac pathology associated with exaggerated hemodynamic responses to VETMEDIN-CA1.
If only radiographic examination is possible, cardiomegaly may be diagnosed in cases where the VHS ≥11.5 and the vertebral left atrial size (VLAS) ≥3.01,2. If radiographic cardiomegaly does not meet both of these criteria, an echocardiogram should be performed prior to the initiation of therapy with VETMEDIN-CA1.
VETMEDIN-CA1 has not been evaluated in dogs receiving concomitant heart medications.
The safety of VETMEDIN-CA1 has not been established in dogs with asymptomatic heart disease caused by etiologies other than MMVD. The safe use of VETMEDIN-CA1 has not been evaluated in dogs younger than 6 months of age, dogs with congenital heart defects, dogs with diabetes mellitus or other serious metabolic diseases, dogs used for breeding, or pregnant or lactating bitches.
Adverse Reactions
In a controlled multi-center field study, 363 dogs with preclinical MMVD (Stage B2 MMVD, 2019 ACVIM Consensus Statement) received at least one dose of VETMEDIN-CA1 (n=182) or the placebo control chewable tablets (n=181) for up to 1563 days. During this long-term study, dogs were followed until the development of congestive heart failure (CHF). Adverse reactions were seen in both treatment groups with many findings associated with the progression of MMVD and comorbidities consistent with the age of the enrolled dogs.The median time to the primary endpoint (development of left-sided CHF or cardiac death/euthanasia) was 38% longer in the VETMEDIN-CA1 group. Despite the longer duration on study, the incidence of reported adverse reactions was similar between treatment groups.
Cough was the most frequently reported adverse reaction. This clinical finding is commonly reported in cases of MMVD and the incidence was similar between treatment groups. Lethargy, inappetence, tachypnea, collapse, arrhythmia, and syncope may also be associated with the progression of MMVD and were reported in dogs receiving VETMEDIN-CA1.
Adverse reactions not related to disease progression in dogs receiving VETMEDIN-CA1 included diarrhea, vomiting, pain, lameness, arthritis, urinary tract infection, and seizure.
Mortality rate, regardless of reason, prior to CHF was similar between the VETMEDIN-CA1 and the control groups.
Contact Information: To report suspected adverse reactions, to obtain a Safety Data Sheet (SDS), or for technical assistance, contact Boehringer Ingelheim Animal Health USA Inc. at 1-888-637-4251. For additional information about reporting adverse drug experiences for animal drugs, contact FDA at 1-888-FDA-VETS or at http://www.fda.gov/reportanimalae.
Information for Dog Owners: Always provide the Client Information Sheet with each prescription and review it with the dog owner or person responsible for care of the dog. Advise dog owners about signs of disease progression and possible adverse reactions with use of VETMEDIN-CA1.
Clinical Pharmacology
Pimobendan is oxidatively demethylated to a pharmacologically active metabolite which is then conjugated with sulfate or glucuronic acid and excreted mainly via feces. The mean extent of protein binding of pimobendan and the active metabolite in dog plasma is >90%. Following a single oral administration of 0.25 mg/kg VETMEDIN-CA1, the maximal mean (± 1 SD) plasma concentrations (Cmax) of pimobendan and the active metabolite were 3.09 (0.76) ng/mL and 3.66 (1.21) ng/ mL, respectively. Individual dog Cmax values for pimobendan and the active metabolite were observed 1 to 4 hours post-dose (mean: 2 and 3 hours, respectively). The total body clearance of pimobendan was approximately 90 mL/min/kg, and the terminal elimination half-lives of pimobendan and the active metabolite were approximately 0.5 hours and 2 hours, respectively.Plasma levels of pimobendan and active metabolite were below quantifiable levels by 4 and 8 hours after oral administration, respectively. The steady-state volume of distribution of pimobendan is 2.6 L/kg indicating that the drug is readily distributed into tissues.
Food decreased the bioavailability of an aqueous solution of pimobendan, but the effect of food on the absorption of pimobendan from VETMEDIN-CA1 is unknown.
In normal dogs instrumented with left ventricular (LV) pressure transducers, pimobendan increased LV dP/dtmax (a measure of contractility of the heart) in a dose dependent manner between 0.1 and 0.5 mg/kg orally. The effect was still present 8 hours after dosing. There was a delay between peak blood levels of pimobendan and active metabolite and the maximum physiologic response (peak LV dP/dtmax). Blood levels of pimobendan and active metabolite began to drop before maximum contractility was seen. Repeated oral administration of pimobendan did not result in evidence of tachyphylaxis (decreased positive inotropic effect) or drug accumulation (increased positive inotropic effect). Laboratory studies indicate that the positive inotropic effect of pimobendan may be attenuated by the concurrent use of a β-adrenergic blocker or a calcium channel blocker.
Reasonable Expectation of Effectiveness: A reasonable expectation of effectiveness may be demonstrated based on evidence such as, but not limited to, pilot data in the target species or studies from published literature.
VETMEDIN-CA1 is conditionally approved pending a full demonstration of effectiveness.
Additional information for Conditional Approvals can be found at www.fda.gov/animalca.
A reasonable expectation of effectiveness for VETMEDIN-CA1 is based on results from a multi-site global field study. The study demonstrated a significant delay in the onset of congestive heart failure in dogs with cardiomegaly and heart murmur secondary to Stage B2 MMVD when treated with VETMEDIN-CA1 at the targeted total daily dose of 0.23 mg/lb (0.5 mg/kg) divided into two administrations approximately 12 hours apart.
A total of 363 dogs across various breeds were randomized to treatment. The resulting population evaluated for effectiveness consisted of 353 dogs receiving either pimobendan (VETMEDIN-CA1, n=178) or control product (placebo chewable tablets, n=175).
Dogs ranged between 6 and 17 years of age and weighed between 9 and 33 lbs at enrollment. Dogs were confirmed to have evidence of Stage B2 preclinical MMVD prior to enrollment, including a systolic heart murmur grade of ≥3/6 and evidence of cardiomegaly, including a VHS >10.5, and echocardiographic evidence of LA/Ao ratio ≥1.6 and LVIDDN ≥1.7.
Dogs were ineligible if they were found to have current or previous evidence of cardiogenic pulmonary edema, clinically significant tachyarrhythmias, cardiac disease other than MMVD, significant systemic disease, evidence of pulmonary hypertension (RA:RV gradient > 65 mmHg), were pregnant or lactating female dogs, or if they were treated with prohibited concomitant medications for 14 or more consecutive days.
The primary outcome evaluated was a composite of the development of left-sided CHF or cardiac-related death or euthanasia. Left-sided congestive heart failure was confirmed by radiographic evidence of cardiogenic pulmonary edema. If a dog died in the absence of evidence of a non-cardiac cause of death, prior to radiographic confirmation of pulmonary edema, it was also considered to have reached the primary endpoint. The study was designed to follow individual dogs for up to 3 years or until disease progression into CHF.
At study termination, 41.6% of the dogs in the VETMEDIN-CA1 group had reached the primary endpoint, compared to 50.3% in the control group. The median time to the primary endpoint was 1228 days in the VETMEDIN-CA1 group compared to 761 days in the control group. Thus, administration of VETMEDIN-CA1 to dogs with Stage B2 preclinical MMVD resulted in the prolongation of the preclinical period by 467 days (15.6 months) compared to dogs receiving control product.
Palatability: In a laboratory study, the palatability of VETMEDIN-CA1 was evaluated in 20 adult female Beagle dogs offered doses twice daily for 14 days. Ninety percent (18 of 20 dogs) voluntarily consumed more than 70% of the 28 tablets offered. Including two dogs that consumed only 4 and 7% of the tablets offered, the average voluntary consumption was 84.2%.
Target Animal Safety: In a laboratory study, pimobendan chewable tablets were administered to 6 healthy Beagles per treatment group at 0 (control), 1, 3, and 5 times the recommended dosage for 6 months. See the table below for cardiac pathology results. The cardiac pathology/histopathology noted in the 3X and 5X dose groups is typical of positive inotropic and vasodilator drug toxicity in normal dog hearts and is associated with exaggerated hemodynamic responses to these drugs. None of the dogs developed signs of heart failure and there was no mortality.
Incidence of Cardiac Pathology/Histopathology in the Six-month Safety Study
|
Severe left ventricular hypertrophy with multifocal subendocardial ischemic lesions |
One 3X and two 5X dogsa |
|
Moderate to marked myxomatous thickening of the mitral valves |
Three 5X dogs |
|
Myxomatous thickening of the chordae tendineae |
One 3X and two 5X dogs |
|
Endocardial thickening of the left ventricular outflow tract |
One 1X, two 3X and two 5X dogs |
|
Left atrial endocardial thickening (jet lesions) in 2 of the dogs that developed murmurs of mitral valve insufficiency |
One 3X and one 5X dog |
|
Granulomatous inflammatory lesion in the right atrial myocardium |
One 3X dog |
a Most of the gross and histopathologic findings occurred in these three dogs
Murmurs of mitral valve insufficiency were detected in one 3X (Day 65) and two 5X dogs (Days 135 and 163). These murmurs (grades II-III of VI) were not associated with clinical signs.
Indirect blood pressure was unaffected by pimobendan at the label dose (1X). Mean diastolic blood pressure was decreased in the 3X group (74 mmHg) compared to the control group (82 mmHg). Mean systolic blood pressure was decreased in the 5X group (117 mmHg) compared to the control group (124 mmHg). None of the dogs had clinical signs of hypotension.
On 24-hour Holter monitoring, mean heart rate was increased in the 5X group (101 beats/min) compared to the control group (94 beats/min). Not counting escape beats, the 3X and 5X groups had slightly higher numbers of isolated ventricular ectopic complexes (VEs). The maximum number of non-escape VEs recorded either at baseline or in a control group dog was 4 VEs/24 hours. At either Week 4 or Week 20, three 3X group dogs had maximums of 33, 13, and 10 VEs/24 hours, and two 5X group dogs had maximums of 22 and 9 VEs/24 hours. One 1X group dog with no VEs at baseline had 6 VEs/24 hours at Week 4 and again at Week 20. Second-degree atrioventricular heart block was recorded in one 3X group dog at Weeks 4 and 20, and in one dog from each of the 1X and 5X groups at Week 20. None of the dogs had clinical signs associated with these electrocardiogram changes.
Treatment was associated with small differences in mean platelet counts (decreased in the 3X and 1X groups), potassium (increased in the 5X group), glucose (decreased in the 1X and 3X groups), and maximum blood glucose in glucose curves (increased in the 5X group). All individual values for these variables were within the normal range. Three 1X and one 5X group dogs had mild elevations of alkaline phosphatase (less than two times normal).
Loose stools and vomiting were infrequent and self-limiting.
Storage Information: Store at 20° to 25°C (68° to 77°F), excursions permitted between 15° and 30°C (between 59° and 86°F).
How Supplied
VETMEDIN®-CA1 (pimobendan) Chewable Tablets:
Available as 1.25 and 5 mg oblong half-scored chewable tablets - 50 tablets per bottle.
NDC 0010-4610-01 - 1.25 mg - 50 tablets
NDC 0010-4612-01 - 5 mg - 50 tablets
References
1 Keene, B., et al. (2019) ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J Vet Intern Med. 33(3):1127-1540.
2 Malcolm, E.L. et. al. (2018) Diagnostic value of vertebral left atrial size as determined from thoracic radiographs for assessment of left atrial size in dogs with myxomatous mitral valve disease. J AM Vet Med Assoc. 253(8):1038-1045.
Marketed by:
Boehringer Ingelheim Animal Health USA, Inc., Duluth, GA 30096
VETMEDIN® is a registered trademark of Boehringer Ingelheim Vetmedica GmbH used under license.
© 2022 Boehringer Ingelheim Animal Health USA Inc. All rights reserved.
180389-001
Revised 03/2022
US-PET-0363-2022
CPN: 1028382.0
3239 SATELLITE BLVD., BLDG 500, DULUTH, GA, 30096
| Telephone: | 800-325-9167 | |
| Customer Service: | 888-637-4251 | |
| Technical Service: | 888-637-4251 | |
| Fax: | 816-236-2717 | |
| Website: | www.boehringer-ingelheim.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
