OvuGel
This treatment applies to the following species:(triptorelin acetate)
100 mcg triptorelin per mL (as triptorelin acetate)
Gel for intravaginal use
INDICATIONS FOR USE:
For the synchronization of time of insemination in weaned sows to facilitate a single fixed-time artificial insemination.
Not approved for use in gilts. Safety and effectiveness have not been evaluated in these animals.
Description
OvuGel® is a thin, clear to slightly hazy gel. Each mL of OvuGel® contains 100 mcg of triptorelin (as triptorelin acetate) for intravaginal administration.
Warnings
 |
WITHDRAWAL PERIOD: No withdrawal period is required when used according to labeling. USER SAFETY WARNINGS: Not for Use in Humans. Keep Out of Reach of Children. The Material Safety Data Sheet (MSDS) contains more detailed occupational safety information. ANIMAL SAFETY WARNINGS: OvuGel® should not be used in sows with obvious reproductive tract abnormalities. |
 |
RECOMMENDATIONS FOR SAFE AND EFFECTIVE USE:
Use of a bottle mount multi-dose applicator is recommended to ensure accurate dosing.
The use of adequate sperm numbers per insemination and proper semen storage, as recommended by the semen or artificial inseminator catheter suppliers, is recommended.
Directions For Use
OvuGel® should be administered intravaginally at approximately 96 hours after weaning. The product should be warmed at room temperature for a minimum of 10 minutes prior to use. Insert the delivery tube into the vagina so that the tip rests 1/2 inch (1-3 cm) posterior to the cervix. Use a separate sheath over the delivery tube for each sow treated. Each sow should receive a single 2 mL dose of OvuGel®. Sows should be inseminated 22 ±2 hours following administration of OvuGel® using standard artificial insemination techniques. Sows should be exposed to a boar during time of insemination. (See below for more detailed directions for OvuGel® administration).
Illustrated OvuGel® Directions for Use
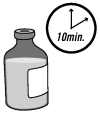
1. Allow the bottle of OvuGel® to warm to room temperature for a minimum of 10 minutes.
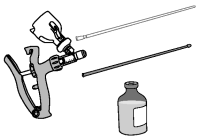
2. Locate the applicator, infusion tube, and protective sheaths to be used. Use of a multi-dose applicator set to deliver 2 mL is recommended.
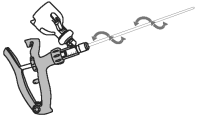
3. Attach infusion tube to applicator.
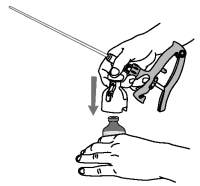
4. Remove foil tab from the bottle top. Invert applicator over upright bottle of OvuGel® and push bottle onto the applicator.
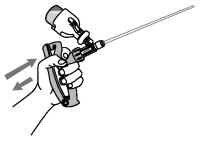
5. Compress the applicator handle fully and then release, allowing applicator chamber to fill with OvuGel®.
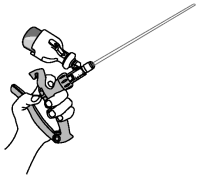
6. Tilt applicator so that infusion tube is pointed up.
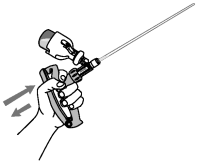
7. Compress and release the applicator handle causing the OvuGel® within the chamber to enter the infusion tube and another dose from the bottle to refill the chamber.
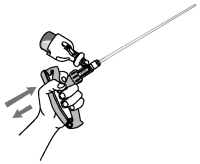
8. Slowly compress and release the applicator handle to displace any air in the infusion tube with OvuGel®. There should now be a full dose in the infusion tube and in the applicator chamber.
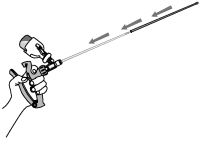
9. Slide the protective sheath over the infusion tube.
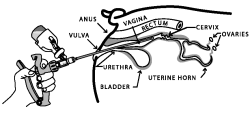
10. Gently and slowly insert the covered infusion tube into the vagina at a slight upper angle (to avoid entry into the urethra) until you encounter mild resistance (the cervix) and then withdraw the infusion tube approximately 1/2 inch (1-3 cm).
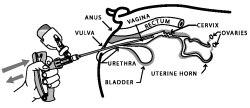
11. Compress and release the applicator handle to discharge the OvuGel® dose and to allow another full dose of OvuGel® to enter chamber.
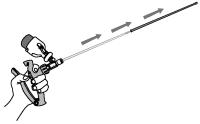
12. Remove the infusion tube and the protective sheath from the vagina. Dispose of the used protective sheath and replace it with a new sheath.
Repeat steps 9 through 12 for each sow receiving OvuGel®.
OvuGel® should be administered within 1 hour of warming. Unused product may be stored under refrigeration for up to 28 days from the date the stopper is first punctured. The bottle stopper may be punctured a maximum of three times during that 28 day period.
STORAGE, HANDLING, AND DISPOSAL:
Store at refrigerator temperature 36-46°F (2-8°C). Excursions permitted to 77°F (25°C) for no more than 5 days during shipping. OvuGel® should be administered within 1 hour of warming; unused portions may be refrigerated immediately after use. The bottle stopper may be punctured a maximum of three times. Discard 28 days after the stopper is first punctured.
How Supplied
OvuGel® is available in 52.5 mL multi-dose bottle (carton of 6).
QUESTIONS/COMMENTS
To obtain an MSDS, for technical assistance, or to report adverse events, contact United-AH II, LLC at 888-842-7218 or www.ovugel.com. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or www.fda.gov/reportanimalae.
Approved by FDA under NADA #141-339
NDC 51233-101-50
OVUGEL is a registered trademark of United-AH II, LLC.
May be covered by one or more patents. For more information go to www.unitedanh.com/patents
United-AH II, LLC, 322 South Main Street, Sheridan, IN 46069
PTK012P
55-1412
Rev. 5/20
CPN: 1726000.3
322 SOUTH MAIN STREET, SHERIDAN, IN, 46069
| Technical Service: | 888-842-7218 | |
| Toll-Free: | 888-842-7218 | |
| Website: | www.ovugel.com | |
| Email: | OvuGel@UnitedAnH.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
