OSPHOS Injection (Canada)
This treatment applies to the following species: Company: Dechra
Company: Dechra
Clodronate disodium injection, 60 mg/mL
DIN 02440776
Veterinary Use Only
THERAPEUTIC CLASSIFICATION: Bisphosphonate
Description
Clodronate disodium is a non-amino, chloro-containing bisphosphonate. Chemically, clodronate disodium is a (dichloromethylene) diphosphonic acid disodium salt and is manufactured from the tetrahydrate form.
Active Ingredients
Each mL contains 60 mg clodronate disodium (as clodronate disodium tetrahydrate), EP. Contains no preservatives.
OSPHOS Injection Indications
For the control of clinical signs associated with navicular syndrome in horses.
Dosage and Administration
Administer by intramuscular injection. Divide the total volume of OSPHOS Injection to be administered evenly into three (3) separate injection sites.The recommended dose is 1.8 mg clodronate disodium/kg body weight (3 mL per 100 kg body weight) up to a maximum dose of 900 mg (15 mL) per horse.
Clinical improvement is most evident at 2 months post-treatment (see EFFICACY). If there is no response to initial therapy, the horse should be re-evaluated.
For horses that initially respond to OSPHOS Injection but do not maintain their clinical improvement for 6 months, OSPHOS Injection may be re-administered at 3 to 6 month intervals based on recurrence of clinical signs. For horses that respond to OSPHOS Injection and maintain clinical improvement for 6 months, OSPHOS Injection should be re-administered after clinical signs recur.
Contraindications
- Do not use OPSHOS Injection in horses with known hypersensitivity to clodronate disodium.
- Do not use this product in horses with impaired renal function or with a history of renal disease.
CAUTIONS:
- The effect of bisphosphonates on the skeleton of growing horses has not been studied, therefore use in horses less than 4 years of age is not recommended.
- The safe use of OSPHOS Injection has not been evaluated in breeding horses or pregnant or lactating mares. Bisphosphonates have been shown to cause fetal developmental abnormalities in laboratory animals and may be excreted in milk. Therefore, this drug should not be used in pregnant or lactating mares, or breeding horses.
- Ensure that horses are adequately hydrated when administering the product.
- Concurrent administration of other potentially nephrotoxic drugs should be approached with caution and renal function should be monitored (see ADVERSE REACTIONS).
- Caution should be used when administering bisphosphonates to horses with conditions affecting mineral or electrolyte homeostasis (e.g., hyperkalemic periodic paralysis, hypocalcemia, etc.) since bisphosphonates affect plasma concentrations of calcium, magnesium and potassium, immediately post-treatment, with effects lasting up to several hours.
- Avoid overdosing with this product as increased bone fragility has been observed in laboratory animals and humans treated with bisphosphonates at high doses or for long periods of time.
Warnings
- Keep out of reach of children.
- Not for use in horses that are to be slaughtered for use in food.
- Care should be taken when handling the product to avoid self-injection, especially by pregnant women. In case of accidental self-injection, consult a physician immediately.
- Avoid contact with skin or eyes. Accidental spillage on the skin or eyes should be washed off with water.
Adverse Reactions
Overview:
As a class, bisphosphonates may be associated with gastrointestinal and renal toxicity. Renal and gastrointestinal adverse reactions may be associated with high plasma concentrations of the drug. The administration of bisphosphonates has been associated with abdominal pain (colic), discomfort, and agitation in horses. Clinical signs usually occur shortly after drug administration and may be associated with alterations in intestinal motility. In horses treated with the recommended dose of OSPHOS Injection, these clinical signs (see Tables 1 and 4) usually began within 2 hours of treatment.
Bisphosphonates inhibit bone resorption and decrease bone turnover which may lead to an inability to repair microdamage within the bone. In humans, atypical femur fractures have been reported in patients on long term bisphosphonate therapy; however, a causal relationship has not been established.
Post-approval experience:
Although all adverse reactions are not reported, the following adverse reaction information is based on voluntary post-approval drug experience reporting. It is generally recognized that this results in significant under-reporting. The adverse events listed here reflect reporting and not necessarily causality. Events are listed in decreasing order of frequency: renal failure, polyuria, polydipsia, abdominal pain, anorexia, lethargy, behavioral disorder (e.g.: head shaking, flehmen response, lip licking), hypercalcemia, urine abnormalities, electrolyte disorder, abnormal test result, hyperactivity, recumbency, hyperthermia, injection site pain, discomfort, muscle tremor, injection site oedema, anemia, and urticaria. In very rare cases, death has been reported as an outcome of the adverse events listed above.
Field trial data:
One hundred forty-six horses (111 OSPHOS Injection, 35 saline control) of various breeds, 4 to 22 years of age, and weighing 367-601 kg (807 to 1322 lb) were included in the field study safety analysis.
Following treatment on Day 0, 10 horses had clinical signs of discomfort or nervousness, cramping, pawing, and/or colic within 2 hours post-treatment. One horse experiencing colic and hives required treatment with flunixin and dexamethasone to resolve clinical signs. In 8 of the 10 horses, 10 to 15 minutes of hand walking resulted in resolution of clinical signs. In one horse, clinical signs resolved without hand walking. Three additional horses experienced lip licking, yawning, and/or head shaking. Adverse reactions occurring within 2 hours post-treatment with OSPHOS Injection or the saline control are summarized in Table 1.
Table 1: Adverse reactions occurring within 2 hours post-treatment
|
Clinical Signs |
OSPHOS Injection (n=111) |
Control (n=35) |
|
Uncomfortable, Nervous, Colic, and/or Pawing |
9.0% (10) |
0% (0) |
|
Lip licking |
5.4% (6) |
0% (0) |
|
Yawning |
4.5% (5) |
0% (0) |
|
Head shaking |
2.7% (3) |
0% (0) |
|
Injection site swelling |
1.8% (2) |
2.9% (1) |
|
Colic requiring treatment* |
0.9% (1) |
0% (0) |
|
Hives/Pruritus |
0.9% (1) |
0% (0) |
* This horse experienced colic and hives and recovered after treatment with flunixin and dexamethasone.
To report suspected adverse drug reactions, contact Dechra at 1-855-332-9334.
INFORMATION FOR HORSE OWNERS: Owners should be advised to observe their horse for at least 2 hours post-treatment for signs of colic, agitation, and/or nervous system abnormalities. If a horse appears uncomfortable, nervous, or experiences cramping post-treatment the owner should be advised to hand walk the horse for 15 minutes until signs resolve. Owners should be advised to contact their veterinarian if the horse displays abnormal clinical signs.
Clinical Pharmacology
Clodronate disodium is a non-nitrogen bisphosphonate that inhibits bone resorption by binding to hydroxyapatite crystals (inhibiting their formation and dissolution), and by exerting direct cellular effects on osteoclasts (inhibiting osteoclast cell function). It has a high affinity for solid-phase calcium phosphate and therefore accumulates in bone. Bound to bone matrix, clodronate disodium enters resorbing osteoclasts, alters their morphology and reduces the number of active osteoclasts, regardless of the cause of osteoclast activity. Clodronate disodium increases bone mass by inhibiting bone resorption and retarding bone turnover.The structural formula of clodronate disodium is:
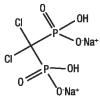
In humans, 60 to 80% of clodronate disodium administered intravenously is eliminated unchanged in the urine and 5% in the feces; the remainder of the dose is distributed to bone. The bone residence time in horses could not be estimated. However, in numerous studies, the half-life of clodronate disodium in rodent bone (long bones and lumbar vertebrae) has been estimated to be months to years.
The pharmacokinetic profile of OSPHOS Injection after a single intramuscular administration in horses diagnosed with navicular syndrome at doses of 300 mg, 900 mg and 1500 mg of clodronate disodium is characterized by rapid absorption of clodronic acid (0.5-0.7 h) and a longer terminal elimination phase. The area under the plasma concentration-time curve (AUC) and maximum concentration (Cmax) increased proportionally with dosage. A dose of 900 mg administered intramuscularly has a plasma half-life of approximately 5.6 ± 2.6 hours, a Cmax of 7.5 ± 1.7 µg/mL and a time to maximum concentration (Tmax) of approximately 0.6 hours.
EFFICACY: A double masked 3:1 randomized, negative control, multi-site field study evaluated the effectiveness of a single dose of 1.4 mg/kg OSPHOS Injection (maximum dose of 900 mg/horse) for the control of clinical signs associated with navicular syndrome in horses. The 146 enrolled horses had a unilateral or bilateral forelimb lameness of Grade ≥ 2 on the AAEP lameness scale (Grade 0 to 5) and a diagnosis of navicular syndrome based on lameness exam, diagnostic nerve blocks, and radiographic signs indicative of the bony changes associated with navicular syndrome. Of the enrolled horses, 111 were treated with OSPHOS Injection and 35 with a saline control. Lameness scores were recorded pre-treatment and on Study Days 28, 56 and 180. There were 114 horses (86 OSPHOS Injection, 28 saline control) included in the statistical analysis. Efficacy was evaluated on Day 56 post-treatment and results are shown in Table 2 below. A horse was considered a treatment success if the lameness grade in the primarily affected limb improved by at least 1 AAEP grade and there was no worsening of lameness grade in the other forelimb on Day 56 post-treatment as compared to the pre-treatment assessment.
Table 2: Day 56 treatment success rate
|
Study Day |
OSPHOS Injection |
Saline |
P Value* |
|
56 |
74.7% |
3.3% |
0.0028 |
* P value and estimated success rates are based on back-transformed mean estimates from the statistical analysis.
Treatment success based on Day 28 and Day 180 lameness scores was also assessed but not statistically analyzed. Results are shown in Table 3 below. The 68 OSPHOS treated horses that were treatment successes on Day 56 were followed to the Day 180 assessment, and the 18 horses that were treatment failures on Day 56 were considered to remain treatment failures at Day 180.
No Day 180 lameness evaluation was performed on these horses. Of the 68 treatment successes from Day 56, 60 horses were evaluable at Day 180. Of these 60 horses, 51 remained treatment successes at Day 180 based on improvement in lameness grade as compared to Day 0. However, 21 of these 60 evaluable horses demonstrated an increase in lameness grade at Day 180 as compared to their Day 56 evaluation.
Table 3: Day 28 and Day 180 treatment success rates
|
Study Day |
OSPHOS Injection |
Saline |
|
28 |
67.4% (60/89) |
20.7% (6/29) |
|
180 |
65.4% (51/78)* |
None evaluable |
* The 60 horses which completed the Day 180 lameness evaluation and the 18 treatment failures from Day 56.
ANIMAL SAFETY: Two studies were conducted to assess the safety of OSPHOS Injection in horses: a six-month target animal safety study (TAS) and a two-phase pilot study evaluating
1) the safety of concurrent use of the recommended 1X dose (1.8 mg/kg) with an NSAID
2) The safety of a single 5X (9 mg/kg) dose.
TAS Study: OSPHOS Injection was administered to 32 healthy adult horses at 0, 1.8, 3.6 and 5.4 mg/kg (0, 1, 2, and 3X the recommended dose) every 28 days for 6 consecutive months. OSPHOS Injection was administered by intramuscular injection with the total volume divided evenly into at least three separate injection sites with a maximum of 15 mL per injection site. Injection site reactions were identified in 10 horses out of 32 (7 treated, 3 controls). Reactions in horses treated with OSPHOS Injection were characterized by soft or firm swellings and resolved within 10 days.
Clinical pathology evaluations showed a dose-related trend for increases in BUN and creatinine post-treatment. A statistically significant increase in the mean BUN concentration was observed in the 2X and 3X groups when compared to the 0X group at 48 hours post-treatment on all six treatment days, but this increase was mild and not considered clinically significant. Statistically significant higher creatinine concentrations were reported for the 3X group compared to the 0X group on Day 0, Day 28, Day 84 and Day 112. However, these statistical differences were not considered clinically significant since the group Least Square means were within the reference range.
A dose-related trend for an increase in potassium was observed for up to 6 hours post-treatment. Individual animal potassium concentrations were within the reference range with the exception of two 3X horses with post-treatment potassium concentrations up to 5.3 mg/dL (reference range: 3-5 mg/dL). Decreases in chloride and increases in glucose, creatine kinase, AST, and ALT were also observed post-treatment. All these values returned to normal by the end of the study. End of study evaluations concluded that bone density (bone mineral concentration) and bone strength (mechanical testing of cortical bone) remained similar between all dose groups.
After the third monthly intramuscular administration in horses of the 3X dose group, a decrease in apparent mean total systemic clearance was seen (0.08 ± 0.02 mL/h), compared to the estimated mean total systemic clearance (0.12 ± 0.02 mL/h) in the horses of the 1X dose group. This decrease resulted in a greater than proportional increase in systemic drug exposure (AUC = 62.49 ± 18.52 h*µg/mL) and plasma elimination half-life (T 1/2 = 2.89 ± 1.33 hours) compared to the AUC and T 1/2 values obtained in horses receiving the 1X dose.
The most common post-treatment observations were clinical signs related to abdominal discomfort (colic) and the central nervous system (yawning, flehmen, tongue rolling, head shaking and neck writhing). As indicated in Table 4 below, the incidence of colic was dose-related. The severity of colic was also dose-related; in the 3X group, clinical signs of colic often persisted after hand walking and horses were often walked more than once. However, no horses in any treatment group received medical treatment.
Colic-related clinical signs began shortly after treatment administration and all horses returned to normal within 5.5 hours post-treatment. Other post-treatment clinical signs were noted in this study and are included in Table 4.
Table 4: Incidence of abnormal clinical signs in the TAS study
|
|
Number of observations per treatment group |
|||
|
Clinical Signs |
0X |
1X |
2X |
3X |
|
Colic* |
4 (8.3%)** |
2 (4%) |
26 (54%) |
45 (94%) |
|
Colic requiring hand walking |
0 (0%) |
0 (0%) |
8 (31%) |
36 (80%) |
|
Yawning |
5 |
17 |
16 |
30 |
|
Flehmen |
0 |
0 |
8 |
2 |
|
Tongue rolling |
1 |
10 |
8 |
10 |
|
Head shaking |
1 |
5 |
3 |
7 |
|
Neck writhing |
0 |
0 |
0 |
6 |
|
Pawing |
4 |
4 |
12 |
23 |
|
Agitation |
1 |
1 |
7 |
10 |
|
Depression |
0 |
2 |
5 |
21 |
|
Muscle fasciculations/Trembling |
0 |
0 |
1 |
4 |
* Signs of colic included repeated lying down and rising, rolling, kicking at the abdomen, stretching of the abdomen and/or other typical signs of abdominal discomfort.
** Percentage incidence for colic was calculated by dividing number of observations by the 48 treatment administrations per group.
Two-phase Study: In Phase I of this study, six horses were administered phenylbutazone orally twice a day at a dose of 4.4 mg/kg on Days 0 to 3, administered OSPHOS Injection at 1.8 mg/kg (1X) by intramuscular injection into 3 sites once on Day 4, and continued on phenylbutazone orally twice a day at a dose of 2.2 mg/kg on Days 4 to 6. In Phase II, after a 15 day washout, the same six horses were administered a single dose of OSPHOS Injection at 9 mg/kg (5X) by intramuscular injection divided evenly into 5 separate injection sites.
In Phase I, three horses had elevations in BUN above the reference range (up to 42 mg/dL; reference range 8-25 mg/dL) 48 hours post-treatment. BUN concentrations returned to normal prior to Phase II of the study.
In Phase II, five out of six horses developed changes in attitude associated with signs of agitation or nervousness including pawing, circling, and tail twitching within 6 minutes of dosing. Four of six horses also developed clinical signs including excessive yawning, flehmen, tongue rolling, head shaking, and head bobbing. All six horses developed mild to moderate muscle fasciculations between 2 and 30 minutes post-treatment. By 30 minutes post-treatment, four out of six horses also developed signs of discomfort and possible abdominal pain including full body stretching, repetitive lying down and rising, and kicking at the abdomen. At approximately one hour post-treatment, one horse exhibited agitation and clinical signs of colic requiring medical therapy. The horse responded to medical therapy and was clinically normal at 7 hours post-treatment. Three out of six horses developed temporary gait abnormalities that included mild to moderate hypermetria, spasticity, or mild ataxia. Four out of six horses developed mildly elevated BUN concentrations by 48 hours post-treatment and one horse had a creatinine concentration slightly above the reference range (2.0 mg/dL; reference range 0.9-1.9 mg/dL) for 12 hours post-treatment.
Storage
Store below 30°C. Single dose vial, discard unused portion.PRESENTATION: OSPHOS Injection is supplied in cartons with each carton containing one clear glass 20 mL vial with 15 mL (900 mg) clodronate disodium (60 mg/mL) per vial.
Patent number: 2,627,942
Dechra Ltd., North Yorkshire, BD23 2RW, UK
Imported and distributed by: Dechra Veterinary Products Inc., 1 Holiday Avenue, East Tower, Suite 345, Pointe-Claire, Quebec, Canada H9R 5N3
Tel.: 1-855-332-9334
617411
CPN: 1786029.2
1 HOLIDAY AVE., EAST TOWER SUITE 345, POINT-CLAIRE, QC, H9R 5N3
| Toll-Free: | 855-332-9334 | |
| Technical Services: | 855-332-9334 Option 1 | |
| Technical Services Email: | technical.ca@dechra.com | |
| Website: | www.dechra.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
