KetoMed (ketoprofen)
This treatment applies to the following species: Company: Bimeda
Company: Bimeda
Sterile Solution, 100 mg/mL
For intravenous use in horses only.
KetoMed (ketoprofen) Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
Ketoprofen is a non-steroidal anti-inflammatory agent of the propionic acid class that includes ibuprofen, naproxen and fenoprofen. Each mL of KetoMed (ketoprofen) contains 100 mg of ketoprofen in an aqueous formulation containing: L-Arginine, 70 mg; citric acid (to adjust pH); benzyl alcohol, 0.025 g (as preservative).
It is packaged in a multiple dose bottle.
Pharmacology
KetoMed is a non-narcotic, non-steroidal anti-inflammatory agent with analgesic and antipyretic properties.
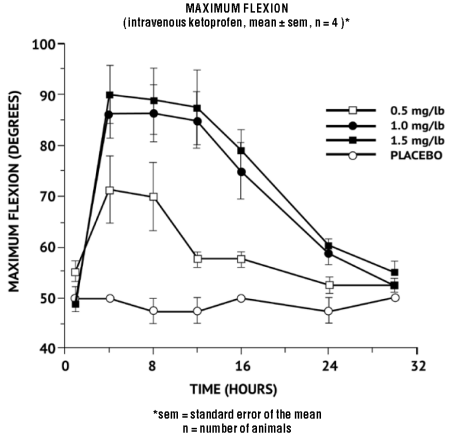
In horses, intravenous dosages of ketoprofen ranging from 0.5 to 1.5 mg/lb resulted in dosage dependent anti-inflammatory effects in the chronic adjuvant carpitis model as depicted in the following graph.
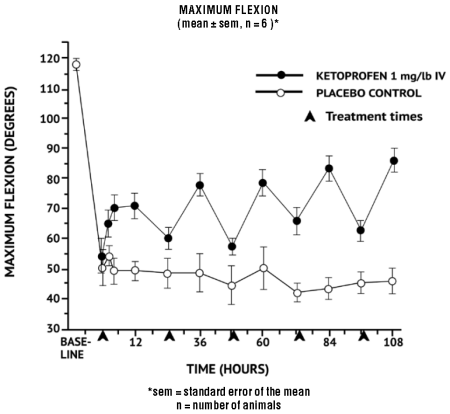
Additional studies using the same model in horses have shown that the effects of ketoprofen are maximal by 12 hours and still measurable at 24 hours after each dosage as depicted in the following graph.
Toxicity
Horses were found to tolerate ketoprofen given intravenously at dosages of 0, 1, 3 and 5 mg/lb once daily for 15 consecutive days (up to five times the recommended dosage for three times the usual duration) with no evidence of toxic effects. In clinical studies, intravenous injection of 1 mg/lb/day for five days resulted in no injection site irritation or other side effects.
At 15-fold overdose (15 mg/lb/day) for five days one of two horses developed severe laminitis, but no gross lesions or histologic changes were observed. The toxic effects observed in the horses given a 25-fold overdose (25 mg/lb/day) for five days included inappetence, depression, icterus, abdominal swelling and postmortem findings of gastritis, nephritis and hepatitis.
KetoMed (ketoprofen) Indication
KetoMed (ketoprofen) is recommended for the alleviation of inflammation and pain associated with musculoskeletal disorders in the horse.
Administration And Dosage
The recommended dosage is 1 mg/lb (1 mL/100 lbs) of body weight once daily. Treatment is administered by intravenous injection and may be repeated for up to five days. Onset of activity is within two hours with peak response by 12 hours.
Contraindications
There are no known contraindications to this drug when used as directed. Intra-arterial injection should be avoided. Do not use in a horse if it has previously shown hypersensitivity to ketoprofen.
KetoMed (ketoprofen) Caution
This product should not be used in breeding animals since the effects of KetoMed on fertility, pregnancy or fetal health in horses have not been determined.
Precautions
Studies to determine activity of KetoMed when administered concomitantly with other drugs have not been conducted. Drug compatibility should be monitored closely in patients requiring adjunctive therapy.
Warning
Do not use in horses intended for human consumption.
Side Effects
During investigational studies, no significant side effects were reported.
To report suspected adverse drug events, for technical assistance, or to obtain a copy of the Safety Data Sheet (SDS), contact Bimeda, Inc. at 1-888-524-6332. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
How Supplied
KetoMed (ketoprofen) 100 mg/mL is available in 50 mL and 100 mL multidose bottles.
Store at 20°C - 25°C (68° - 77°F); excursions permitted between 15°C - 30°C (59°F - 86°F). Do not freeze.
Use within 28 days of first puncture.
Approved by FDA under ANADA # 200-625
Manufactured for:
Bimeda, Inc., Le Sueur, MN 56058
www.bimeda.com
KetoMed™ is a trademark of Bimeda, Inc.
8KET013 Rev. 12/20
|
50 mL Multiple Dose Bottle |
1KET007 |
8KET010 Rev. 12/20 8KET009 Rev. 12/20 |
|
100 mL Multiple Dose Bottle |
1KET008 |
8KET012 Rev. 12/20 8KET011 Rev. 12/20 |
CPN: 1399138.0
Div. Cross Vetpharm Group, Ltd.
ONE TOWER LANE-SUITE 2250, OAKBROOK TERRACE, IL, 60181
| Telephone: | 630-928-0361 | |
| Fax: | 630-928-0362 | |
| Website: | www.bimedaus.com | |
| Email: | us-sales@bimeda.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
