Hymatil (Canada)
This treatment applies to the following species: Company: Modern Veterinary Therapeutics
Company: Modern Veterinary Therapeutics
Tilmicosin Injection, USP
DIN 02431912
Each mL contains 300 mg of tilmicosin
VETERINARY USE ONLY
Sterile
For use in cattle and lambs only.
An antibiotic for subcutaneous administration in cattle and lambs.
Active ingredient - Each mL contains:
|
Tilmicosin |
300 mg |
Non-medicinal ingredients - Each mL contains:
|
Propylene glycol |
250 mg |
|
Phosphoric acid |
q.s |
|
Water for injection |
q.s |
Hymatil Indications
Hymatil is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia (Pasteurella) haemolytica and Pasteurella multocida and for the reduction of morbidity associated with bovine respiratory disease (BRD) in feedlot calves, caused by Mannheimia (Pasteurella) haemolytica and Pasteurella multocida, during the first 30 days in the feedlot, when administered at the time of arrival. Hymatil is indicated for the treatment of pneumonic pasteurellosis in lambs associated with Mannheimia (Pasteurella) haemolytica.
Dosage and administration: Inject subcutaneously in cattle. Inject subcutaneously in lambs greater than 15 kg body weight only. Administer a single subcutaneous injection of 10 mg tilmicosin/kg body weight (1 mL per 30 kg/1.5 mL per 100 lbs.). Do not inject more than 25 mL per injection site.
If no improvement is noted within 48 hours, the diagnosis should be reconfirmed.
NOTE: Swelling at the subcutaneous site of injection may be observed but is transient and usually mild. To limit the development of antimicrobial resistance, Hymatil should only be used as an arrival treatment when BRD has been diagnosed. One or more of the following factors typically characterizes calves at high risk of developing BRD: commingling of calves from multiple sources; extended transport times (that may have included few if any rest stops) and excessive shrink; continued exposure to extremely wet and cold weather conditions and/or exposure to wide ambient temperature changes from origin to arrival; stressful arrival processing procedures such as castration and dehorning; recent weaning and poor vaccination status.
Contraindication: Do not administer intravenously. Intravenous injection in cattle and lambs has been fatal. Do not use in automatically powered syringes.
Caution: Do not administer to swine. Injection of tilmicosin in swine has been fatal.
Warnings
Treated animals must not be slaughtered for use in food for at least 28 days for cattle and 36 days for sheep after the latest treatment with this drug. Do not use in lactating dairy cattle. Tilmicosin may cause sensitization by skin contact. Avoid contact with eyes or skin. Wash hands after use. To limit the development of antimicrobial resistance, Hymatil should only be used as an arrival treatment in feedlot calves when BRD has been diagnosed. Keep out of reach of children.|
Human warning: Not for human use. Human injection has been associated with fatalities. Do not use in automatically powered syringes. Exercise caution to avoid accidental self-injection. In case of human injection, consult a physician immediately and apply ice or cold pack to injection site. Do not apply ice directly to skin. For emergency medical information call 1-800-268-9017. |
|
Note to physician: The cardiovascular system is the target of toxicity and should be monitored closely. Cardiovascular toxicity may be due to calcium channel blockade. In dogs, administration of intravenous calcium offset tilmicosin-induced tachycardia and negative inotropy (decreased contractility) within approximately 20 minutes. Dobutamine dose-dependently partially offset the negative inotropic effects induced by tilmicosin in dogs, but did not have an effect on the increased heart rate caused by tilmicosin. β-adrenergic antagonists, such as propranolol, exacerbated the negative inotropy of tilmicosin in dogs. Epinephrine potentiated lethality of tilmicosin in pigs. Epinephrine is contraindicated. This antibiotic persists in tissues for several days. |
Description
Hymatil injection is a preconstituted solution of the antibiotic tilmicosin. Each mL contains 300 mg of tilmicosin activity; 25% propylene glycol; phosphoric acid as needed to adjust pH; water for injection, q.s.
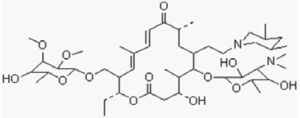
Tilmicosin is produced semi-synthetically and is a member of the macrolide class of antibiotics.
Activity: Hymatil has an in vitro antibacterial spectrum that is predominantly Gram positive with activity against certain Gram negative microorganisms. Activity against several mycoplasma species has also been detected.
|
Microorganism |
Minimum Inhibitory Concentration (µg/mL) |
|
Pasteurella multocida |
6.25 |
|
Mannheimia (Pasteurella) haemolytica |
3.12 |
|
Haemophilus somnus |
6.25 |
|
Staphylococcus aureus |
0.78 |
|
Streptococcus agalactiae |
3.12 |
|
Actinomyces pyogenes |
0.024 |
|
Clostridium perfringens |
3.12 |
|
Clostridium sordellii |
3.12 |
|
Fusobacterium necrophorum |
3.12 |
|
Escherichia coli |
≥50.00 |
|
Salmonella typhimurium |
≥50.00 |
|
Mycoplasma dispar |
0.097 |
|
Mycoplasma bovirhinis |
0.024 |
|
Mycoplasma bovoculi |
0.048 |
|
Acholeplasma laidlawii |
0.024 |
One hundred thirty-four of 139 (96.4%) Mannheimia (Pasteurella) haemolytica isolates tested were inhibited by less than or equal to 3.12 µg/mL tilmicosin.
With Pasteurella multocida Carter Type A of bovine origin, 53/54 (98.2%) isolates tested were inhibited by less than or equal to 12.5 µg/mL tilmicosin and 39/54 (72.2%) by less than or equal to 6.25 µg/mL.
In addition to its direct antibacterial action, tilmicosin may exert an anti-inflammatory effect in the lung by increasing neutrophil apoptosis and reducing the release of pro-inflammatory mediators. However, the clinical significance of this effect is unknown. In clinical trials, BRD treatment success with tilmicosin was usually characterized by rapid reduction in body temperatures, less severity of clinical signs, better weight gains and reduced mortality.
Toxicology: The cardiovascular system appears to be the target of toxicity in laboratory animals and domestic livestock administered tilmicosin by oral or parenteral routes. The primary cardiac effects are increased heart rate (tachycardia) and decreased contractility (negative inotropy).
Cardiovascular toxicity may be due to calcium channel blockade. Upon injection subcutaneously, the acute median lethal dose (mLD) of tilmicosin in mice is 97 mg of activity per kg and in rats is 185 mg/kg of body weight. Given orally, the mLD of tilmicosin is 800 mg/kg and 2250 mg/kg in fasted and nonfasted rats respectively. No compound-related lesions were found at necropsy.
In dogs, intravenous calcium offset tilmicosin-induced tachycardia and negative inotropy, restoring arterial pulse pressure within approximately 20 minutes. Dobutamine dose-dependently partially offset the negative inotropic effects induced by tilmicosin in dogs, but did not have an effect on the increased heart rate caused by tilmicosin. β-adrenergic antagonists, such as propranolol, exacerbated the negative inotropy of tilmicosin in dogs.
In monkeys, a single intramuscular dose of tilmicosin at 10 mg/kg caused no signs of toxicity. A single dose of tilmicosin at 20 mg/kg caused vomiting and 30 mg/kg caused the death of the only monkey tested.
In swine, intramuscular injection of tilmicosin at 10 mg/kg caused increased respiration, emesis and a convulsion, 20 mg/kg resulted in mortality in 3 of 4 pigs and 30 mg/kg caused the death of all 4 pigs tested. Injection of tilmicosin at 4.5 and 5.6 mg/kg intravenously followed by epinephrine, 1 mL (1:1000) intravenously 2 to 6 times, resulted in death of all pigs injected. All pigs given 4.5 mg/kg and 5.6 mg/kg tilmicosin intravenously with no epinephrine survived. These results suggest intravenous epinephrine may be contraindicated. Results of genetic toxicology studies were all negative. Results of teratology and reproduction studies in rats were negative. The no effect level in dogs after daily oral doses of tilmicosin for up to one year is 4 mg/kg of body weight.
In cattle, subcutaneous doses of tilmicosin at 10, 30 and 50 mg/kg of body weight, each injected at 72 hour intervals for three times, did not cause any deaths. As expected, edema at the site of injection was noted. In cattle, the only lesion observed at necropsy was minimal myocardial necrosis in the 50 mg/kg tilmicosin group. Subcutaneous doses of tilmicosin at 150 mg/kg injected at 72 hour intervals resulted in deaths. Edema was marked at the site of injection. Minimal myocardial necrosis was the only lesion observed at necropsy. Deaths of cattle have been observed with a single intravenous dose of tilmicosin at 5 mg/kg of body weight.
In lambs, single subcutaneous doses of tilmicosin up to 150 mg/kg of body weight did not cause death. Deaths of lambs have been observed with a single intravenous dose of 7.5 mg/kg body weight.
Pharmacokinetics: A single subcutaneous injection of tilmicosin at 10 mg/kg of body weight in cattle resulted in peak tilmicosin levels within one hour and detectable levels (0.07 µg/mL) in serum beyond 3 days. However, lung concentrations of tilmicosin remained above the tilmicosin MIC (95% of 3.12 µg/mL) for Mannheimia (Pasteurella) haemolytica for at least three days following the single injection. Serum tilmicosin levels are a poor indicator of total body tilmicosin. The lung/serum tilmicosin ratio in favour of lung tissue appeared to equilibrate by three days post injection at approximately 60. In a study with radioactive tilmicosin, 24% and 68% of the dose was recovered from urine and feces respectively over 21 days.
How Supplied
Hymatil is supplied in multidose 100 and 250 mL amber bottles containing 300 mg of tilmicosin activity per mL.Storage
Store at 30°C (86°F) or below. Protect from direct sunlight. Discard unused product after 28 days of first broaching the vial.Manufactured for: Modern Veterinary Therapeutics, LLC, Miami, Florida 33186 - USA
www.modernveterinarytherapeutics.com
info@modernveterinarytherapeutics.com
Orders & Product information: Call 1 888 590-9839
Revision date: 01 June 2020
CPN: 1354009.4
261065 WAGON WHEEL WAY, ROCKY VIEW COUNTY, AB, T4A 0T5
| Telephone: | 407-852-8039 | |
| Toll-Free: | 888-590-9839 | |
| Website: | www.modernveterinarytherapeutics.com | |
| Email: | info@modernveterinarytherapeutics.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
