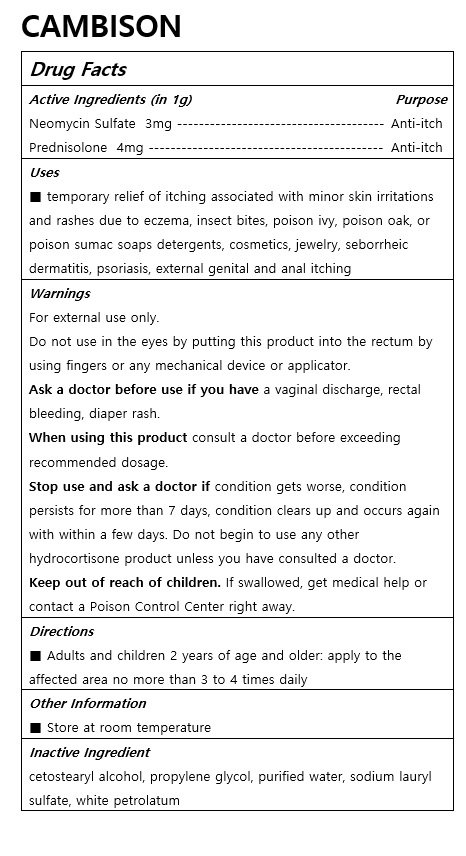
CAMBISON
Dosage form: ointment
Ingredients: PREDNISOLONE 4mg in 1g, NEOMYCIN SULFATE 3mg in 1g
Labeler: OASIS TRADING
NDC code: 72689-0016
Medically reviewed by Drugs.com. Last updated on Nov 27, 2023.
Neomycin Sulfate, Prednisolone
■ temporary relief of itching associated with minor skin irritations and rashes due to eczema, insect bites, poison ivy, poison oak, or poison sumac soaps detergents, cosmetics, jewelry, seborrheic dermatitis, psoriasis, external genital and anal itching
Keep out of reach of children
■ Adults and children 2 years of age and older: apply to the affected area no more than 3 to 4 times daily
For external use only.
Do not use in the eyes by putting this product into the rectum by using fingers or any mechanical device or applicator.
Ask a doctor before use if you have a vaginal discharge, rectal bleeding, diaper rash.
When using this product consult a doctor before exceeding recommended dosage.
Stop use and ask a doctor if condition gets worse, condition persists for more than 7 days, condition clears up and occurs again with within a few days. Do not begin to use any other hydrocortisone product unless you have consulted a doctor.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
cetostearyl alcohol, propylene glycol, purified water, sodium lauryl sulfate, white petrolatum
For external use only
| CAMBISON
neomycin sulfate, prednisolone ointment |
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
| Labeler - OASIS TRADING (689991468) |
| Registrant - OASIS TRADING (689991468) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| OASIS TRADING | 689991468 | manufacture(72689-0016) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.