What are Black Box Warnings?
Boxed warnings (also known as black box warnings) are the strictest warning that can be issued for a drug by the Food and Drug Administration (FDA). First implemented in 1979, they are there to alert doctors to a potentially serious side effect of a medicine or to restrictions on the use of a medicine.
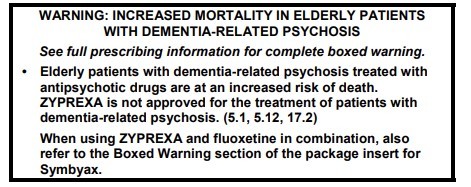
The warning or restriction must be formatted with a box or border around the text and prominently displayed on the package insert or any promotional material connected with that medicine, including the manufacturer's website. They do not have to be included on the label that is attached to the medicine when it is dispensed in a pharmacy.
Not all drugs carry a boxed warning; however, all drugs have general warnings and precautions related to their use. For most people, the only way to know if there is a boxed warning associated with a drug is if your doctor tells you about it.
Boxed warnings are meant for the prescriber, which is usually a doctor. On seeing the boxed warning, the prescriber has the duty to inform the person taking the medicine of the warning and the risks associated with that medicine. They do not always mean that it is unduly risky to take the medicine. In fact, more than 40% of patients outside of a hospital are likely to be administered at least one medication that carries a black box warning within a 30-month period. But it is important to know that some boxed warnings are for side effects that are rare and only happen with a frequency of 1 in 10,000 or 1 in 100,000.
Sometimes the FDA requires a manufacturer of a drug with serious safety concerns to develop a REMS (this stands for a Risk Evaluation and Mitigation Strategy) to mitigate the risk of a product with a boxed warning. A REMS is a drug safety program that is designed to reinforce certain behaviors and actions that support the safe use of that medicine.
For example, Zyprexa Relprevv is a long-acting injectable antipsychotic medication that may be used to treat adults with schizophrenia. A serious reaction, called post-injection delirium sedation syndrome, can occur within three hours of Zyprexa Relprevv in less than 1 percent of people, and the drug carries a boxed warning about this syndrome.
The REMS associated with Zyprexa Relprevv states that the drug can only be administered in certified health care facilities that are able to observe patients for at least three hours and provide appropriate medical care in case of an adverse event. Only a few medications require a REMS.
What types of warnings are boxed?
For a drug to receive a boxed warning, it must fulfill one of the following criteria:
- A very serious side effect that could potentially cause death or permanent disability has been associated with the drug
- A very serious side effect has been associated with the drug, but the severity can be minimized by following certain guidelines such as excluding people with certain other medical conditions from taking the drug, monitoring, or not taking certain medicines with the drug
- Mandatory restrictions exist for prescribing the drug. For example, doctors wanting to prescribe it must undergo compulsory training, or the drug can only be administered in certain settings, such as under supervision or in an inpatient ward.
With most boxed warnings, there is still some uncertainty with regards to the actual risk; however, the FDA tends to err on the side of caution and boxed warnings are reasonably common.
Weighing up the risks versus benefits
As with all medicines, the benefits of taking that medicine must outweigh the risks. This still applies to drugs with a boxed warning. Even though a side effect may be severe enough to carry a boxed warning, your doctor considers that the benefits of the drug for you are worth the risk of taking that drug.
For example, leflunomide (Arava) is a medicine used in the treatment of Rheumatoid Arthritis. It carries a boxed warning for embryo-fetal toxicity (this means it can cause harm to an unborn baby if the mother takes leflunomide) and for hepatotoxicity (which is liver damage). This means that women who are pregnant or likely to become pregnant, or people with a history of liver problems or taking other medications that could cause liver problems are most at risk of these reported effects. Everybody else is considered at low risk.
Another important factor doctors need to consider is what could happen if you did not take the drug. If we use the example of leflunomide again, uncontrolled inflammation that occurs as a result of not taking leflunomide could permanently damage several organs in the body, such as the heart and the lungs. It really is a balancing act and doctors use their medical knowledge to decide if that drug is right for you or not. Doctors take boxed warnings seriously and do not usually consider a drug with a boxed warning until other treatments have been tried and do not work, or if treatment for your condition is urgent.
More information
The best person to talk to about a boxed warning is your doctor. Ask them to detail the risks and benefits that pertain to you, if there are any alternatives, and let them know how YOU feel about taking that drug.
There may be certain things you can do to mitigate the severity or probability of that adverse effect happening or certain symptoms you should look out for. Ultimately, your willingness to take a medicine with a boxed warning comes down to your tolerance for risk, the perceived benefits of the medicine, and your current state of health. It should be a joint decision made between you and your doctor once you completely understand all the pros and cons.
Do not be afraid to ask questions and take some time to think it through.
Sources
- O'Shea T. 10 Black Box Warnings Every Pharmacist Should Know 15 March 2016 pharmacytimes.com/contributor/timothy-o-shea/2016/03/10-black-box-warnings-every-pharmacist-should-know
- An FDA Guide to Drug Safety Terms
https://www.drugs.com/fda-consumer/an-fda-guide-to-drug-safety-terms-52.html
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
